8.0 Bioavailability to Human Populations
Consideration of bioavailability in human health exposure can significantly impact cleanup objectives and, consequently, the feasibility of particular remedial options. Bioavailability as it pertains to human exposure involves estimating the potential exposure to humans via food consumption using biouptake methods, most of which have been discussed in previous chapters, and can also include estimating the level of contaminant absorption by humans once exposure occurs.
As indicated in the generic CSM presented in Section 2.1, humans can be exposed to contaminated sediment both directly via wading or swimming in impacted areas and indirectly via consumption of organisms or plants that have accumulated contaminants from sediment. Because of the potential for biomagnification, low contaminant concentrations in sediment may result in unacceptable risks to humans consuming fish or shellfish. Compared with other human exposure pathways, fish consumption tends to be a common risk driver scenario for sediment sites. Human exposure to COPCs may also occur via ingestion of piscivorous wildlife, primarily game birds that may have some level of COPCs in their tissue as a result of their dietary consumption of impacted fish or shellfish. Ingestion of affected plants such as wild rice that are cultivated or grow wild in contaminated areas (see St. Regis case study in Section 7.4) can also be a complete exposure pathway.
The focus of this section is how the bioavailability of contaminants in freshwater and marine sediments affects human exposures. Other variables associated with evaluating human exposure, such as the extent or frequency of exposure, are topics for the risk assessment and are not considered here. Figure 8-1 shows a general flow diagram for this consideration.
8.1 Direct Contact
Human exposures risks may arise from dermal contact with sediment or incidental ingestion of sediment during activities such as swimming, beach use, dockyard work, boat and marine equipment operation/repair, diving, etc. A first-cut screening for this pathway can be completed by comparing bulk sediment concentrations to published federal or state SSLs (e.g., USEPA’s “Regional Screening Levels for Chemical Contaminants at Superfund Sites,” USEPA n.d. “Regional”). Since sediment direct contact exposures are generally so much lower than those for soil, if sediment concentrations are found to be below these criteria, no further assessment is required. If these preliminary screening criteria are exceeded, site-specific screening values may be developed by modifying exposure variables to reflect the differences between soil and sediment exposures.
Human exposure to sediment differs from exposures to soil due to potential differences in the physicochemical properties of the two media, as well as the unique scenarios under which these exposures to sediments occur. However, because studies addressing human exposure to sediment are limited, assessments for sediment often default to scenarios and uptake values published for soil in either state or federal guidance. Bioavailability factors addressing various COPCs in soil (the adherence of sediments to skin, dermal absorption efficiency, and gastrointestinal absorption efficiency) have been empirically derived for some classes of COPCs and are available in USEPA’s RAGS (USEPA 2004c). The Agency for Toxic Substances and Disease Registry toxicological profiles (www.atsdr.cdc.gov/toxprofiles/index.asp) also provide contaminant-specific absorption information. USEPA (2004c) also addresses bioavailability considerations for dermal contact with contaminated sediment. Factors that decrease bioavailability include water content, complexation in the water column, and the degree of sediment presence above water (submerged sediments typically wash off and do not pose an exposure concern). Several of the categories for skin adherence (e.g., wet soil) could apply to exposed sediment.
USEPA allows a rapid, inexpensive in vitro gastric simulation test to generate site-specific bioavailability factors following the ingestion of lead in soils (http://www.epa.gov/superfund/health/contaminants/bioavailability/guidance.htm). Although sediment is a different medium, it is close enough to soil to justify that the mechanism of uptake in the gastrointestinal tract would be comparable. In cases where one or more COPCs drive an unacceptable risk outcome after using a default bioavailability factor and assuming sediment uptake is similar to soil, the user may want to conduct additional literature research or testing and/or engage in discussions addressing the bioavailability of the COPCs in sediments with the project team.
Individual USEPA regions may also have guidance on how to derive site-specific bioavailability factors. For example, Region 8 conducted several studies on the bioavailability of arsenic in a variety of soils found in Colorado (USEPA n.d. “Bioavailability”). Based on these studies, it recommends a conservative relative bioavailability factor of 0.5 for arsenic from contaminated soil (i.e., 50% of the soil arsenic, relative to sodium arsenate, will be absorbed from the gastrointestinal tract). An example of an animal study confirming this factor using site-specific sediment, which could be applied to any jurisdiction, is described at the end of this chapter.
8.2 Fish and Shellfish (Aquatic Invertebrate) Ingestion
In evaluating whether dietary exposure to humans is an important pathway, the type of contaminant should be considered. USEPA (n.d. “Persistent,” www.epa.gov/pbt/pubs/aboutpbt.htm) has identified 12 PBT constituents. Some states and regions, such as Texas and the Pacific Northwest (RSET 2009), have established lists of contaminants for which the bioaccumulative pathway must be considered. In general, however, bioaccumulation concerns should be limited to selected classes of organics (pesticides, PCBs, dioxins) and mercury.
Evaluation of sediment-associated contaminant accumulation by fish and shellfish has been described in Chapters 4– 6. This section describes how the accumulation evaluation is incorporated into human health exposure estimation.
8.2.1 Development of Screening Values
Generic screening levels for sediment for the fish ingestion pathway can be back-calculated from acceptable tissue levels (ATLs) that are estimated assuming a particular fish ingestion rate and human toxicity factors. In some cases, an established reference, such as a fish tissue advisory, may also be used. As previously defined (Section 5.2.3.1), BSAF or BAF values are often used to back-calculate a bulk sediment concentration associated with achieving an ATL for each individual COPC:
screening level value = foc(ATL/(BSAF)(fL))
where foc is the fraction of total organic carbon in the sediment and fL is the fraction of lipid content in the edible tissue. For development of generic screening levels, default assumptions for foc and fL are used. However, organic carbon and lipid fractions can be used to adjust screening levels based on site-specific bioavailability. Where site-specific data are unavailable for these two parameters, they may still be adjusted to better represent site-specific conditions by using published data for similar conditions (e.g., sediment type, edible fish species present, etc). Two sources of published BSAFs are USACE (n.d. “BASF”) and USEPA (n.d. “BASF”).
In some cases the site-specific SLVs are higher to the extent that exposure via a certain pathway is determined to be below a level of concern (see Text Box 8‑1).
ODEQ has established a recreational human fisherman generic sediment SLV of 0.019 mg/kg for hexachlorobenzene. The ATL in fish tissue is 0.0058 mg/kg, the estimated BSAF is 0.105 kg OC/kg lipid, and the default values for fish lipid fraction and sediment TOC fraction are 0.03 and 0.01, respectively. However, game fish at the site have a lipid fraction of 0.02, and sediment sampling indicates an average TOC of 0.10. Using the site-specific values in the SLV equation:
site-specific SLV = (0.10 × 0.0058 mg/kg) / (0.105 kg OC/kg lipid) × 0.02) = 0.28 mg/kg
Thus, applying site-specific information raises the screening value 15-fold.In cases where SLVs are being used to estimate food source concentrations, they might also be modified based on the contaminant concentration that is available in the sediment pore water, which in some cases may reflect the bioavailable contaminant fraction. It may be determined that the pathway for bioaccumulation involves the step of dissolution (bioaccessibility) into adjacent pore water before uptake into an organism’s tissue can occur. There is significant uncertainty in this assumption as it is not clear what processes occur during digestion that may make sorbed contaminants bioavailable.1
Pore-water concentrations can be measured directly by several tools described in Appendix C. Pore-water concentrations can also be estimated using assumptions about EqP to sediment OC (organic compounds) or AVS (divalent inorganic metals) in the sediment. A variety of models have been presented in Chapter 4 that could be used to apply pore-water results to adjust the fraction of contaminant concentration that is bioavailable in the human health exposure pathway. Direct measurement of pore water has also been detailed in Chapter 4.
8.2.2 Direct Tissue Analysis of Fish/Shellfish
In many cases, human exposures to COPCs from consumption of fish and shellfish are estimated using actual measured concentrations of COPCs in tissue. Direct measurement of COPC concentrations in tissue reduces the uncertainty regarding bioavailability in sediments. Depending on the size of the impacted zone relative to the home range of the receptor species analyzed, it may be difficult to directly correlate concentrations of COPCs in sediment with fish tissue COPC concentrations. Impacts from contaminant sources upstream or downstream from the site may also complicate the relationship between site impacted sediment and fish concentrations. However, field measurements generally provide more accurate estimates of bioaccumulation than do published BSAFs, which tend to be generic and overly conservative.
Collection and measurement of fish and shellfish (invertebrates) are discussed in Chapter 5; however, several additional factors are pertinent to determining the representativeness of these data when collected for use in human health evaluations:
- Site fidelity—Sampling should occur from areas where fishing/collection is known or predicted to occur.
- Species—Species should be sampled that serve as food sources for human populations. Size and/or age restrictions on fish that can be caught may apply to certain species.
- Tissue—Edible portions should be analyzed. Often only fish fillets are consumed, which tend to accumulate lower concentrations of contaminants than other portions (e.g., organs, bones, fatty deposits, etc.) of the fish. It is important to understand the local population and their potential practices since some populations consume or use entire fish.
- Preparation method—Different levels of contaminant loss are associated with different fish-preparation methods. For example, frying fish in a pan may retain more contaminants than grilling, in which fat is allowed to drip.
Depending on the size of the impacted zone relative to the home range of the receptor species analyzed and/or impacts from contaminant sources upstream and downstream from the site, it may be difficult to directly correlate concentrations of COPCs in sediment with fish tissue COPC concentrations. However, field measurements generally provide more accurate estimates of bioaccumulation than do published BSAFs, which tend to be generic and overly conservative. As has also been discussed in previous sections, fish tissue values can be estimated or obtained through laboratory bioaccumulation tests or use of caged species placed at the site.
8.3 Wildlife Ingestion
Fish are typically the primary species of concern when assessing human health risks for bioaccumulative contaminants in sediment. However, consideration should also be given to potential exposures that may occur via ingestion of other wildlife. Ducks, for instance, can be exposed to contaminated sediment via incidental ingestion of sediment and through dietary ingestion of aquatic vegetation and benthic and/or pelagic organisms. The primary method for assessing this pathway is to obtain information on the dietary habits of the species of concern and use data collected as described in Chapter 6 to estimate concentrations that may accumulate in the target wildlife species. Exposures are then estimated based on these dietary components.
8.4 Plant Ingestion
Similarly, humans may be exposed to sediment-associated contamination via ingestion of plants that have been grown in the contaminated area (e.g., seaweed, wild rice) or crops in which dredged spoil was used as a soil amendment. Chemical bioavailability to plants is challenging to predict given the wide array of plant species and their varying chemical accumulation potential, the varying chemical conditions that can affect chemical bioavailability (e.g., pH and redox conditions), and the general lack of bioaccumulation models with specificity to plant tissue types (e.g., roots, seeds, or leaves). In practice, plant tissue concentrations are not frequently evaluated on contaminated sediment sites since generally other pathways (e.g., fish consumption) dominate the exposure to COPCs. Site-specific measurements of plant tissue concentrations can quantitatively determine the degree to which chemicals are accumulating in plants and the degree to which this exposure pathway may contribute to overall sediment-related exposures. In the event that site-specific plant tissue measures are proposed, the refinement of target species and tissues needs to occur based on an understanding of the exposure pathway of concern. The plant tissues collected and processing methods used define the use of these data. For example, it may be appropriate to evaluate whole plant concentrations (e.g., algae), or it may be appropriate to evaluate plant parts separately (e.g., roots and shoots), depending on which plant parts are anticipated to be consumed by a particular receptor species. If human consumption of the plant product is a concern, the sample needs to be prepared in a manner that is consistent with how the product is consumed, which may include washing, peeling, and/or cooking. Other sample preparation steps may be appropriate if exposures to wildlife are being evaluated.
8.5 Application of Bioavailability Tools in the Human Health Pathway
8.5.1 Industri-plex Superfund Site, Woburn, Massachusetts
The Industri-plex case study illustrates the use of both in vitro and in vivo testing to assess the relative bioavailability (RBA) of arsenic in sediment to humans who could potentially be exposed through recreational contact. The Industri-plex site was once occupied by the former Merrimac Chemical Co., which was once the nation’s leading producer of lead arsenate, the main insecticide used in apple orchards in the 19th century. Prior to completion of the human risk assessment, an arsenic bioavailability study was performed to increase the level of site-specificity being incorporated into the quantification of sediment risks. The bioavailability study consisted of two phases: an in vitro screening phase followed by an in vivo bioavailability assessment. The in vitro extraction test was performed on a total of 12 fine-sieved sediment samples obtained from four locations along the Aberjona River (www.epa.gov/region1/superfund/sites/industriplex/213091Appendix6part10.pdf). The in vitro test served as a screening tool to identify specific selection criteria for use in identifying a smaller number of samples to carry through to the in vivo assessment. The in vitro test measured the amount of arsenic that dissolves in a reactor that simulates the stomach fluid of humans, and the amount of arsenic that solubilized after one hour was used as a preliminary indicator of the in vivo RBA.
Using the results of the in vitro test to identify two appropriate sediment test materials, an in vivo bioavailability assessment was conducted wherein immature swine were fed dough balls containing sediment test materials at weights set to equal target doses of 300, 600, and 900 µg/day. Control animals were fed equivalent doses of sodium arsenate. Samples of urine were collected from each animal for three consecutive 48-hour periods on days 6/7, 8/9, and 10/11 of the study. Laboratory analyses were submitted in a blind fashion, and measurements accounted for all forms of arsenic (i.e., As[III], As[V], and methylated species). The RBA of arsenic in the sediment samples was calculated by dividing the absolute bioavailability (ABA, which is the amount absorbed/amount ingested) of the test sediments by the ABA of the sodium arsenate. The RBAs of the site sediments were 37% and 51%, respectively. The risk assessment toxicity factors were adjusted accordingly using the more conservative relative bioavailability factor of 0.51 (i.e., USEPA Integrated Risk Information System (IRIS) reference dose was divided by 0.51 and the cancer slope factor multiplied by 0.51). Thus, the study results reduced the estimated human health risks by half.
8.5.2 Johnson Lake
Johnson Lake covers an area over 18 acres and is directly connected to the Whitaker Slough, which in turn flows to the Columbia Slough, a quiescent waterway located south of the Columbia River. A number of environmental investigations conducted at the site have indicated elevated levels of PCBs, metals, and PAHs in lake sediment resulting from runoff from surrounding properties. Sediment samples collected throughout the lake indicated that PCBs were present at concentrations (57–1040 ppb) that exceeded screening levels (0.39 ppb) for protection of human health based on fish consumption. To assess the level at which PCBs were being accumulated in fish, a fish tissue sampling effort was undertaken. Tissue concentrations (90% upper confidence level of 870 ppb whole body fish) exceeded the ATL for human health (4.7 ppb). Whole body tissue samples were appropriate in this case as there was evidence that local populations were using whole fish in stews. Bioavailability was indirectly measured in this assessment through the calculation of a site-specific BSAF. The site-specific BSAF was then used to calculate a protective sediment concentration of PCBs.
ODEQ concluded that sediment contamination in Johnson Lake poses an unacceptable risk to human health based on the risk associated with ingestion of PCB-contaminated fish. Human health-based RAOs for this site were as follows:
- eliminate potential hot spots in Johnson Lake sediments by managing sediment to the extent practicable to reduce the average PCB concentration in sediment by approximately 72%
- prevent human consumption of fish with tissue concentrations greater than 0.003 µg/kg PCB congener 126
The areas with highest PCB concentrations were proposed for removal, reducing average sediment concentrations by 72%. This reduction in average PCB concentrations in the lake is expected to result in close to an order of magnitude reduction in potential cancer risk associated with fish ingestion. Once this initial reduction is achieved through active remediation efforts, such as the removal of sediments in the southern portions of the lake, natural recovery mechanisms along with upland source control measures are expected to further reduce concentrations to protective levels. The ROD for the Johnson Lake site can be viewed at http://www.deq.state.or.us/Webdocs/Forms/Output/FPController.ashx?SourceId=1311&SourceIdType=11.
![]()
1One way to evaluate whether this is an important step for biouptake is to estimate or directly measure pore-water concentrations of COPCs and then develop a regression between pore-water concentrations and measured food-source concentrations. If there is a stronger correlation with estimated or measured pore-water concentrations than with bulk sediment concentrations, then dissolution into pore water is likely an important step in biouptake.
2As discussed in Chapter 2, a detailed discussion of risk assessment is beyond the scope of this document; however, Table 2-2 provides references for conducting ecological/human health risk assessments.



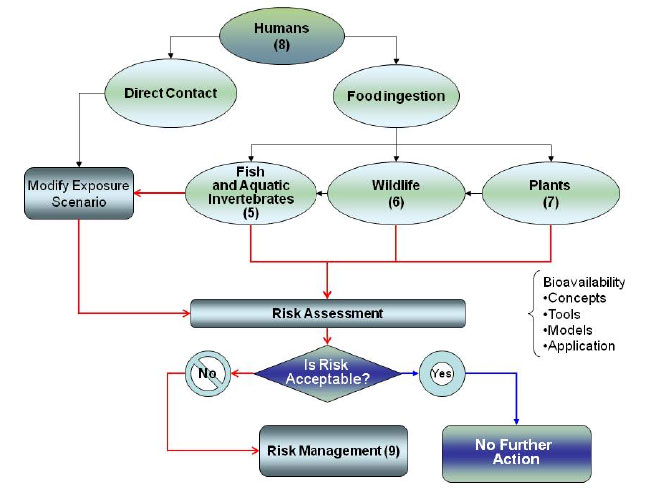 Figure 8-1. Human health decision diagram.
Figure 8-1. Human health decision diagram.