7.0 Bioavailability in Plants
Determination of direct plant toxicity from plant tissue concentration measurements is generally not a factor in ecological risk assessment and management. More often, measured plant tissue concentrations are intended to be used in the food chain exposure assessment for humans and wildlife.
Plants serve as primary producers in ecosystems. At its most extreme, plant toxicity can result in loss of this function (e.g., unvegetated areas). Secondary effects may include erosion, habitat loss, or food loss for other trophic levels. However, because of their sessile nature (with the exception of aquatic algae), plants have evolved unique chemical exclusion (e.g., at the root zone) and compensatory (e.g., metals chelation) mechanisms that allow them to control chemical bioavailability and to survive in environments that could be toxic to other types of life. For example, miners historically used observations of specific metal-tolerant or hyperaccumulating plants (i.e., metallophytes) to determine locations of mineral-rich soils (Baker, Brooks, and Reeves 1988).
There is also a wide array of plant sensitivities to chemicals. Lewis (1995) has shown that some freshwater plants, such as periphyton (aquatic algae that grow on rocks), are more sensitive to many chemicals than are fish and invertebrates. Other plants are less sensitive and may help remediate contaminated areas (i.e., phytoremediation) (Seidel et al. 2004). Plants can, for example, create and sustain oxygenated root-zone habitat suitable for communities of chemical-degrading bacteria, or they may uptake and sequester chemicals in specific parts of the plant that may then be harvested. Less-sensitive plants are frequently an important part of reclamation and restoration projects.
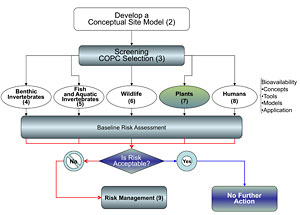
This chapter describes how bioavailability of contaminants in freshwater and marine sediments to plants can be incorporated into ERAs. Figure 7-1 shows a general flow diagram for the bioavailability tests for the plant pathway. Two methods of measuring bioavailability in plants are discussed in the following sections:
- chemical measurement of bioavailability in the specific plant tissues
- plant uptake BAFs used to estimate plant tissue concentrations
No sediment chemical screening levels have been developed for the protection of aquatic plants. Therefore, this section reviews SSLs (as a surrogate for sediment) and water screening levels (as a surrogate for sediment pore water) that are intended to be protective of plants, while acknowledging that sediment properties are distinct from soil (e.g., percent moisture and OC content). It is recognized that these thresholds for terrestrial plants may be significantly different than those for aquatic systems but may be an indicator of magnitude of potential harm.
USEPA has two sources of screening levels that address the potential for plant toxicity at hazardous waste sites. The first are ecological soil screening levels (EcoSSLs, www.epa.gov/oswer/riskassessment/ecorisk/ecossl.htm) compiled for the most common COPCs reported in recent RODs at Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) sites (derived by workgroups with participants representing state, federal, consulting, industry, and academic stakeholders). The second contains chemical stressor concentration limits (CSCLs) published as part of the data collected for USEPA’s Hazardous Waste Identification Rule (USEPA 1999a). The Department of Energy’s ORNL has also developed Toxicological Benchmarks for Screening Contaminants of Potential Concern for Effects on Terrestrial Plants (Efroymson etal. 1997, www.esd.ornl.gov/programs/ecorisk/documents/tm85r3.pdf). These screening levels are intended to be concentrations that cause de minimis effects on plant receptors and can be used to identify COPCs in soils requiring further evaluation in a baseline ERA. Most soil screening values are derived using conservative assumptions, and the resulting benchmarks are therefore also conservative (some may be less than site background levels).
7.2 Biological Approaches
7.2.1 Bioassays
Plant bioassays have been designed to determine phytotoxicity using a wide variety of aquatic plant species (Gorsuch et al. 1991). These tests have predominantly used terrestrial crop plants (e.g., lettuce) or freshwater aquatic algae and plants (e.g., duckweed).
Advantages
- Controlled conditions so the effects of the site sediment on chemical bioaccumulation in a specific plant species can be more directly assessed.
- Reduced variability in bioaccumulation, which has relevance to comparisons to available plant tissue screening levels.
Disadvantage
- Species tested and/or the concentrations measured may not be relevant to site-specific plants or the diet of wildlife/ humans.
USEPA (1994a) examined the utility of using a freshwater rooted vascular plant (Hydrilla verticillata, or water thyme) toxicity test. Out of 43 combinations of test species and endpoints, the Hydrilla endpoint of root length ranked third in terms of sensitivity, but other endpoints in this species were found to be less sensitive: shoot length (22nd), dehydrogenase activity (26th), chlorophyll a (28th), and peroxidase (43rd). Despite promising results with the root length endpoint, no standard regulatory test methods have been developed for Hydrilla.
Reviews of available toxicity tests and estuarine specific field and laboratory studies using plants are contained in Gorsuch et al. (1991) and Lytle and Lytle (2001), respectively. Currently, research is being conducted to develop aquatic plant species test methods including procedures for water milfoil (Myriophyllum—a draft method exists and has been evaluated by USACE), eelgrass (Vallisnaria), water weed (Elodea), and other species.
Most evaluations of plant toxicity involve the comparison of soil (or sediment) COPC concentrations to chemical parameters of concern concentration believed to cause toxicity in plants. The plant screening levels discussed in Section 7.1I have limited ability to predict plant toxicity, since the chemical bioavailability associated with specific site conditions is not known. However, a few sources of plant tissue thresholds of toxicity do exist for specific crops, as shown in Table 7-1.
7.2.2 Bioaccumulation
Plant bioaccumulation tests are less frequently implemented than toxicity test, and the only standard test methods use upland species, either human food crop test species (USEPA 1996a–m) or preferred confined disposal facility test species (USACE 2002). Appendix C lists plant toxicity and bioaccumulation test procedures and plant bioaccumulation field studies.
Table 7-1.
Plant tissue toxicity reference values (ppm dry weight)
Analyte |
Mature
Leaf Toxic Levela |
Threshold for Yield Reduction |
50%
Yield Reductione |
||
Plant
Specificb |
General c,d |
||||
| Arsenic | 5–20 | NAf | 20 | NA | NA |
| Cadmium | 5–30 | 8 (barley) | 15 | 5 | NA |
| Chromium | 5–30 | NA | 10 | NA | 5.9 (com) |
| Cobalt | 15–50 | NA | 20 | NA | NA |
| Copper | 20–100 | 19 (barley) 21 (lettuce) 6 (rape) 11 (ryegrass) 11 (wheat) |
20 | 10 | 40 (com) 60 (bush bean) |
| Lead | 30–300 | NA | 35 | NA | NA |
|
a Kabata-Pendias and Pendias 1984. b Beckett and Davis 1977. c Davis, Beckett, and Wollan 1978. d Macinoll and Beckett 1985. e Chang, Granato, and Page 1992. f Data not available. |
7.3 Modeling Plant Bioavailability and Bioaccumulation
Plant bioaccumulation models based on empirical data have been extensively reviewed by ORNL (Bechtel Jacobs 1998a) as well as USEPA for the protection of human health and wildlife as part of the development of SSLs and EcoSSLs, respectively (USEPA 1996a–m, 2005a). These models represent the potential transfer of chemicals from soil to plants and, in the absence of sufficient data specific to aquatic plant chemical accumulations from sediment, they are the only viable surrogate for estimating chemical bioavailability and uptake in aquatic plants. Additionally, in the screening level ecological risk assessment (SLERA) protocol guidance, USEPA published summaries of soil-to-plant BAFs and considers these applicable as sediment-to-plant BAFs (USEPA 1999b). Despite the recommendation, we recognize that the physicochemical characteristics of sediment differ markedly from soil and may influence the bioavailability of chemicals in sediments to aquatic vegetation in ways that cannot be predicted. The primary difference is clearly the presence of continuous overlying water, which affects sediment redox potential, chemical bioavailability, and the types of biological communities that are present. The soil bioaccumulation models are intended to be used in environments where the organic matter content is less than 10% and pH is in the range 4.0–8.5.
Advantage
- Potentially less costly than direct measurement.
Disadvantages
- Estimates are uncertain and not site or tissue specific (e.g., roots, shoots, leaves, and berries) with most plant BAFs developed for specific soil conditions.
- Sediment conditions, particularly sediment redox potential, are known to affect chemical bioavailability to plants.
BAFs and regression equations have been used to predict soil-to–terrestrial plant bioaccumulation. For the earlier evaluation performed by USEPA (1996m), only BAF estimates were available from the literature for the crops of interest. At the time of a later evaluation (USEPA 2005b), some regression equations were available and were preferred over BAFs as long as the equation was significant (i.e., the slope differed significantly from 0 @ p = 0.05) and the coefficient of determination (R2) was ≥0.2. Bechtel Jacobs (1998b) found that uptake factors did not lead to the best estimates of plant tissue concentrations.
Appendix C summarizes BAFs and bioaccumulation equations for inorganics available from USEPA SSL (USEPA 1996m), USEPA SLERA protocol (USEPA 1999b), and USEPA EcoSSL (USEPA 2005b) guidance. There are no specific sediment conditions (e.g., grain size and TOC) that can be used as modifying factors for a BAF. BAFs are applied to bulk sediment concentrations. Where a BAF is not available, USEPA EcoSSL guidance suggests using a default BAF of 1.
Equations for individual organic compounds were not available at the time of the SSL guidance (USEPA 1996m) but were reported in the USEPA SLERA protocols (USEPA 1999b) and EcoSSL guidance (USEPA 2005b) for select organic analytes (see Appendix C).
USACE has also developed a Plant Uptake Program (PUP version 1.0 EEDP-04-12) that can be used to estimate the bioaccumulation of inorganic chemicals from freshwater dredged material (sediment or soil media, Folsom and Houck 1990). This program was developed through the collaboration of the U.S. Army Engineer Waterways Experiment Station (WES, renamed Engineering Research and Development Center) Environmental Laboratory and Purdue University to evaluate wetland or upland dredged material disposal as a beneficial use option. The plant species used for this estimation was Cyperus esculentus (yellow nut grass), and uptake estimates were based on data collected 1977–1989 by WES and its contractors. These data showed that heavy metals extracted from sediment using diethylenetriaminepentaacetic acid (DTPA) correlated well with plant uptake. The program uses regression techniques (ordinary least squares) to estimate the upper 90% confidence interval of the concentrations taken up by plants.
The use of DTPA and other sediment extraction methods to determine the bioavailable fraction of contaminants to plants has been reviewed by the NRC (2003). Other chemical extracts used to estimate bioavailability to plants include ammonium bicarbonate-DTPA, dilute hydrochloric acid, the Mehlich-3 mixture (composed of acetic acid, ammonium nitrate, nitric acid, ammonium fluoride, and EDTA), and the Mehlich-1 mixture (composed of dilute solution of hydrochloric and sulfuric acids, NRC 2003). A limitation on the use of specific extract procedures is that correlations with actual plant concentrations may be poor if the plants evaluated are different than the species used in the development of the extraction procedures or if the matrix conditions are different than the conditions of those evaluated during the development of the extraction procedure (e.g., pH).
Chemical bioavailability to plants is challenging to predict given the wide array of plant species and their varying chemical accumulation potential, varying chemical conditions that can affect chemical bioavailability (e.g., pH and redox conditions), and the general lack of bioaccumulation models with specificity to plant tissue types (e.g., roots, seeds, or leaves). In practice, plant tissue concentrations are not frequently evaluated on contaminated sediment sites since generally other pathways (e.g., fish consumption by wildlife or humans) dominate the transfer of chemical contaminants in food chains. Site-specific measurements of plant tissue concentrations can quantitatively determine the degree to which chemicals are accumulating in plants and the degree to which this exposure pathway may contribute to overall sediment-related exposures. In the event that site-specific plant tissue measures are proposed, the refinement of target species and tissues needs to occur based on an understanding of the exposure pathway of concern. The plant tissues collected and processing methods used define the use of these data. For example, it may be appropriate to evaluate whole plant concentrations (e.g., algae), or it may be appropriate to evaluate plant parts separately (e.g., roots and shoots), depending on which plant parts are anticipated to be consumed by a particular receptor species. If human use of the plant product (i.e., fruit consumption) is a concern, the sample needs to be prepared in a manner that is consistent with how the product is consumed, which may include washing, peeling, and/or cooking. Other sample preparation steps may be appropriate if exposures to wildlife are being evaluated.
7.4 Application of Bioavailability Tools in Risk Assessment and Risk Management
Direct assessment of plant bioaccumulation potential can provide valuable information to a risk assessment that may otherwise be highly uncertain if based on the application of simple bioaccumulation models such as BAFs. Determination of direct plant toxicity from plant tissue concentration measurements is generally not a factor in ecological risk assessment and management. More often, measured plant tissue concentrations are intended to be used in the food chain exposure assessment for humans and wildlife. The St. Regis Paper Co. site in Minnesota provides an example of how select plant parts and processing methods were used in a human and ecological exposure assessment. The St. Regis Paper Company Superfund site, located in the city of Cass Lake, Minnesota, is the former location of a wood-preserving facility that operated 1957–1985 (USEPA n.d. “St. Regis”). Creosote and pentachlorophenol (PCP) are the two wood preservatives used throughout the operational history. The principal COPCs at this site are PCDDs and PCDFs, unintended by-products associated with pentachlorophenol, that include the identified carcinogen 2,3,7,8-tetrachlorodibenzo-p-dioxin. Additional COPCs evaluated at this site include PCP, PAHs, PCBs, pesticides, and metals. The site occupies 125 acres within the exterior boundaries of the Leech Lake Band of the Ojibwe Indian Reservation adjacent to Pike Bay and Cass Lake (both freshwater bodies). Wild rice production is very important to the economy and culture of the Leech Lake Ojibwe people, and their reservation produces more wild rice than any other reservation in the state. Accordingly, it was important at this site to evaluate the potential human health risk posed by the consumption of wild rice. Human health risk was evaluated on processed rice samples, which involved finishing (i.e., parching, removing hulls, and winnowing) the rice. Unprocessed rice samples were used in the evaluation of ecological (avian and mammalian receptor) risk and to understand the effect processing had on rice sample concentrations. USEPA has not made these risk assessments available on the Internet, so the degree to which chemical concentrations were accumulated by rice (processed or unprocessed) cannot be reported. A cleanup decision has not been reached for this site, but a ROD is anticipated.



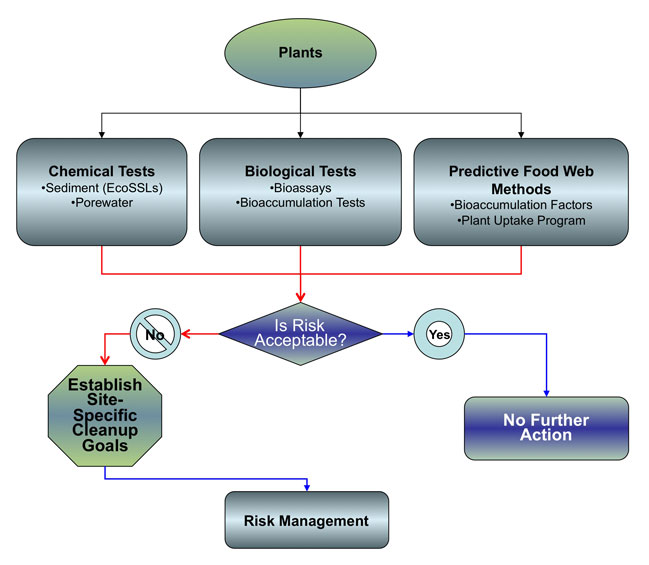 Figure
7-1. Plant Community Decision Diagram
Figure
7-1. Plant Community Decision Diagram