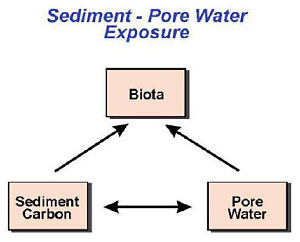
4.0 Bioavailability to Benthic Invertebrates
This chapter presents the tools for assessing bioavailability to benthic macroinvertebrates that live in or on sediment. This pathway is often the first pathway assessed when sediment chemical screening levels are exceeded (see Chapter 3). Beyond the screening criteria, additional investigations may be conducted to determine whether the COPCs are affecting the benthic community. These investigations can include additional sediment chemistry analyses, bioassays, macroinvertebrate community surveys, or geochemical measurements that take into account site-specific conditions that may influence COPC bioavailability.
Do bulk sediment concentrations of COPCs indicate potential for impact to benthos based on applicable SQGs?
If no, then probability of impact to benthos may be low.
If yes, then use weight-of-evidence approach that can include bulk sediment chemistry, laboratory-based toxicity tests, and/or macroinvertebrate surveys to gain information by the following:
- substituting pore-water measurements for bulk sediment chemistry:
- estimate EqP or SEM – AVS
- direct analysis through active or passive sampling
- estimating bioavailability from laboratory-based toxicity tests
- conducting laboratory bioaccumulation exposures
- conduct toxicity identity evaluation (TIE) to determine contaminant responsible for toxicity
Benthic invertebrates are relatively sedentary organisms that inhabit or depend on the sediment environment to sustain life functions. Because they largely live on (epibenthic) or in (infaunal) the sediment, they are sensitive to both short- and long-term changes in sediment and water quality. Benthic invertebrates are frequently used as environmental indicators of biological integrity because they are found in most aquatic habitats; are of a size permitting ease of collection; reflect water quality conditions or sustainability of ecosystem components; are consumed by a wide range of wildlife species, including fish, amphibians, reptiles, birds, and mammals; and can be used to identify of impaired conditions (USEPA 1989d).
Assessment of exposure and bioavailability for benthic organisms depends on where they live and how they derive nutrition. Infaunal organisms living within the sediment can be exposed to COPCs through pore water, through ingestion of contaminated sediments or diet, or through COPCs released into the overlying water. (For some benthic organisms, such as clams, respiration and feeding are accomplished by siphoning the overlying surface water.) Exposure of epibenthic organisms is principally through ingestion of contaminated sediments or diet and COPCs released into overlying water.
This section identifies and describes the tools and measures that are available to assess bioavailability for the benthic invertebrate pathway. Text Box 4-1 presents a generalized roadmap for assessing bioavailability to benthos, which will be discussed throughout this section.
4.1 Tools for Assessing Benthic Invertebrate Bioavailability
Benthic invertebrates may be prey for higher-level organisms and potentially transfer COPCs through the food web (Figure 4-1). If bulk sediment concentrations exceed state SQGs, the traditional procedure for evaluating the effect of sediment-based COPCs on benthic organisms is through the Sediment Quality Triad. The SQT is a weight-of-evidence approach that integrates results from sediment chemistry, aquatic toxicity testing, and benthic community analysis. MacDonald and Ingersoll (2002) provide an example of one method of interpreting the results of the SQT (see Table 4-1). For some states, sediment chemistry and aquatic toxicity testing may be given more weight than benthic community analysis because benthic metrics are often difficult to interpret, particularly for staff without formal training in benthic ecology. Additionally, there are other factors besides chemistry (e.g., grain size, depth, TOC, salinity, discharge) that may markedly affect the number and spatial distribution of benthic invertebrates in sediment and thus introduce bias during routine field sampling.
In addition to bulk sediment chemistry, additional measures that can be used to assess whether sediment quality may be affecting benthic macroinvertebrates (or the benthic community as a whole) include the analysis of pore-water chemistry and macroinvertebrate tissue residue analysis (e.g., bivalves).
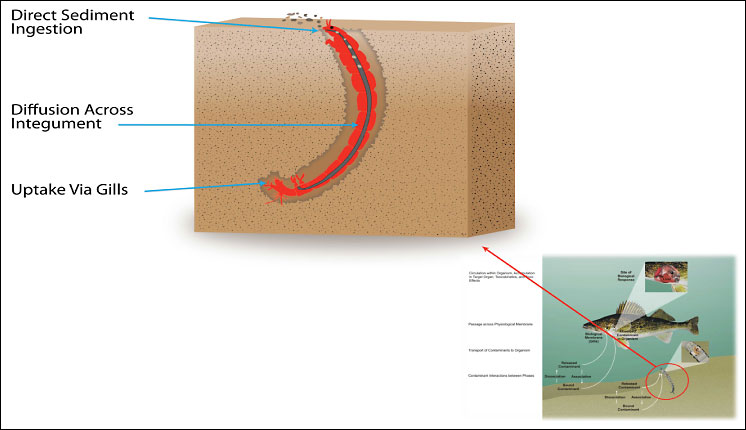
Figure 4-1. General bioavailability processes in the benthic pathway in sediments. (adapted after NRC 2003)
Table 4-1. Contingency table of possible outcomes using the Sediment Quality Triad (Source: USEPA 2002d)
| Possible outcome | Sediment chemistrya | Toxicity testa | Benthic communitya | Possible conclusions |
| 1 | + | + | + | Impact highly likely |
| 2 | - | - | - | Impact highly unlikely |
| 3 | + | - | - | Impact unlikely |
| 4 | - | + | - | Impacts possible |
| 5 | - | - | + | Impacts unlikely |
| 6 | + | + | - | Impact likely |
| 7 | - | + | + | Impact likely |
| 8 | + | - | + | Impact likely |
a+ indicates classified as affected; - indicates classified as unaffected |
Sediment chemistry provides information on the types and levels of COPCs that may be causing an effect on the benthic community. Sediment toxicity testing provides a direct measure of the potential risk posed by the sediment. It does not provide any information about the source of that risk. Benthic community analysis may or may not be helpful in determining effects of COPCs. This parameter is highly variable and subject to influences other than COPCs (e.g., substrate or lack of food). It should be noted, however, that benthic macroinvertebrates have been assigned “pollution tolerance” scores (Section 4.1.2.2) so that the presence of a certain family, genus, or species can often inform the observer as to how disturbed the community is by human influence (Barbour et al. 1999).
- Chemical measures provide contaminant concentrations in sediments, water, or tissue.
- Biological measures provide inference about contaminant bioavailability.
- Predictive measures use models to estimate chemical and biological measurements that can be used to assess contaminant bioavailability.
Bioavailability concepts, tools, models, and/or applications should be considered when examining the physicochemical and risk-related aspects of sediments. Given the conservative risk assessment practice that assumes many exposure parameters may be “worst case” and independent of each other, simply assuming that a COPC is “100% bioavailable” will most likely result in an overestimation of risk and thus an uninformed decision. While there are suites of conventionally employed tests that are routinely used in federal and state site characterization programs, recent advanced tools and measures have made it possible to explore with more accuracy the degree to which a COPC is bioavailable by correlating the chemical activity with toxicological response in benthic invertebrates. Figure 4-2 presents a schematic to assist in examining tools, models, and/or applications of bioavailability within the benthic macroinvertebrates pathway. The various tools are linked to the section of the guidance that provides a description and application of the method.
The tools discussed in this section have been classified as chemical, biological, or predictive. As a matter of convenience, these subdivisions help to classify these tools and their application as described in Table 4-2.Table 4-2
Table 4-2. Approaches used to assess bioavailability in the benthic pathway
| Measure | Conventional Approach | Advanced Approach | |
| Chemical Measures | Bulk sediment chemistry |
|
|
Groundwater (App. C-T1) |
|
|
|
| Surface water (App. C-T7) |
|
|
|
| Tissue residue analysis (App. C‑T5) |
|
|
|
| Biological Measures | Toxicity testing (freshwater, App. C‑T3 and marine, C‑T3) |
|
|
| Macroinvertebrate survey (App. C‑T6) |
|
|
|
| Predictive Measures | Partitioning models (App. C-T4 and C‑T8) |
|
|
4.1.1 Chemical Approaches
Chemical approaches are those techniques that provide COPC concentrations in sediments, pore water, surface water, and, if applicable, groundwater and tissue residues. Appendix B presents the major classes of chemicals found at contaminated sediment sites and specific bioavailability considerations for these classes of compounds. Appendix C provides more detailed descriptions and links to the tools described below. The following sections describe the concepts of assessing bioavailability with each tool or method and their application within the benthic pathway.
4.1.1.1 Bulk sediment chemistry
Contaminated sediment sites require, at a minimum, an analysis of the total (bulk) concentration of the COPC(s) in sediment, as this measure is used for defining the nature and extent of site-related contamination, as well as for comparison to screening-level benchmarks, background comparisons, and/or local conditions (see Chapter 3). Screening of bulk sediment chemistry concentrations to SQGs is sometimes sufficient for risk management decisions, particularly when sites are small and/or there are prescriptive state sediment procedures for screening evaluations. Where background and relevant SQGs are exceeded, pore-water analysis, toxicity testing, and/or benthic macroinvertebrate surveys can be conducted to assess the actual impact to the sediment community. Confounding factors associated with benthic community assessments may include non-site-related factors (e.g., grain size, depth, TOC, salinity, discharge).
Bulk sediment chemistry is also used in predictive models of bioavailability to benthos and/or subsequent accumulation by fish, as well as to evaluate the potential exposure to higher organisms including humans. Horizontal (areal extent) and/or vertical (depth interval) assessments of bulk sediment chemistry with physical descriptions are needed for completing nature and extent evaluations under most site investigation programs, assessing COPC fate and transport, and planning and implementing remedial alternatives. With regard to spatial sampling, the scale (and budget) of the site determines the number of samples required to meet any predetermined degree of statistical power (Gilbert 1987). For vertical sampling, the depth of the bioactive zone is typically defined as 0–6 inches for freshwater sediment and a 0–1 foot interval for estuarine and marine habitat (USEPA 2001b). Most remote sampling grab devices, such as a clamshell type, typically obtain the 0–6 inch interval. Site specific conditions, however, may require alternative considerations of depth intervals (e.g., scouring).
Specific chemical and physical parameters to be measured when evaluating benthic exposure are site and/or program specific. These include the COPCs and may also include TOC, sediment grain size, ∑SEM – AVS parameters (sulfides), pH, salinity or alkalinity, ammonia, and redox potential. These parameters, their relevance, and test methods are described in USEPA 2001c (https://projects.itrcweb.org/contseds-bioavailability/References/collectionmanual.pdf). Methods for the collection and analysis of COPCs in bulk sediment are described in multiple federal and state documents (USEPA n.d. “Measurement,” MacDonald and Ingersoll 2002, NFESC 2003a, NJDEP 1998, WDOE 2008, RSET 2006).
4.1.1.2 Pore-water and groundwater measures
Undisturbed sediment pore-water COPCs tend towards equilibrium with the solid-phase COPC concentrations (i.e., EqP, see Section 4.1.3.1 for explanation). Pore-water contamination, however, may also occur from groundwater discharge into the interstitial space.
Measuring chemicals in groundwater that discharges into sediments has conventionally been done with groundwater monitoring wells or piezometers positioned along the shoreline and inferring or predicting attenuation and discharge concentrations with groundwater models (Winter 2002). More recently methods have been developed to sample tidal and subaqueous groundwater discharges to a water body (Chadwick and Hawkins 2008, Chadwick et al. 2003, Duncan et al. 2007a, 2007b). These tools include intertidal seep sampling, piezometers, and diver-deployed diffusion samplers. Text Box 4-2 illustrates several examples of samplers used to sample pore water (including passive groundwater samplers). For a complete list of available sampling tools of indirect and direct measurement of COPCs in pore water, see Appendix C-T1 (direct pore-water sampling devices) and C-T2 (indirect pore-water sampling devices). Those sections provide more details and descriptions of the tools and measures applicable to measuring COPCs in groundwater and pore water. USEPA (2008a) also provides information on the evaluation of the groundwater to sediment transport pathway.
The principal routes of exposure for benthic infauna are through ingestion of sediment or direct exposure to contaminated pore water. Generally, there is a good correlation between biological effects and pore-water concentrations but not with total sediment concentrations (Di Toro et al. 1991). The bioavailability of a COPC from pore water is theoretically expressed as the “truly” dissolved phase and as such is separate from the COPC associated with suspended particulates and DOC (although the latter plays a role in phase equilibrium for organic compounds). Since the toxicity to benthic organisms is generally correlated to pore-water concentrations, the relationship of pore-water concentrations to established NRWQC (see USEPA n. d. “National”) should theoretically provide a measure of the potential toxicity of the COPC. While the most common form of assessing COPC impacts is the comparison of bulk sediment concentrations to sediment quality criteria, this measurement does not provide information on site-specific bioavailability of the COPC. As a line of evidence assessing exposure to the benthic community, some states approve of the assessment of site-specific toxicity to benthos from sediments by the determination of the pore-water concentrations from bulk sediment concentrations using EqP theory. Recent advances in using solid-phase sampling devices (e.g., SPME, POM) would suggest that direct sampling of pore water would considerably increase accuracy while reducing the cost/benefit ratio of the site investigation (Hawthorne and Hawthorne 2009).
Field-deployable subaqueous sampling systems include both active and passive samplers. SPME fibers are thin silica fibers coated with an organic polymer. Polyethylene devices (PEDs) are polyethylene sheets. HOCs partition from the pore water onto the polymer/ plastic. SPMDs are polyethylene tubes that may be filled with laboratory-grade distilled water or triolein and deployed in the water column or sediments. Sediment “peepers” rely on a diffusion gradient across a dialysis membrane into a pure sampling liquid. With SPMDs, contaminants of interest passively diffuse and come into equilibrium in materials in the bag. The Trident probe is a simple, direct-push system equipped with temperature, conductivity, and water sampling probes and can collect water at specific depths. The UltraSeep Meter can be used to make continuous, direct measurements of the groundwater seepage rate using an ultrasonic flow meter (Chadwick et al. 2003, Chadwick and Hawkins 2008, Smith et al. 2003). The GORE® Module is a waterproof, vapor-permeable GORE-TEX® membrane tube containing hydrophobic adsorbents. Dissolved contaminants partition and diffuse through the membranes to the adsorbent (ITRC 2007). (SPME, PED, and SPMD photos courtesy of Dr. R. Burgess, USEPA; Trident and UltraSeep photos courtesy of Dr. B. Chadwick, U.S. Navy Space and Naval Warfare Systems Center; GORE Module photo courtesy W. L. Gore.)

Pore-water measures are useful to apply when existing site data, based on bulk sediment chemistry and possibly aquatic toxicity testing or benthic community analysis, suggest that a specific contaminant may be responsible for an observed toxic response. Measurements of contaminants in pore water involve more advanced tools than evaluating total sediment concentrations, principally to collect the pore water and/or contaminants that are then measured by standard analytical techniques. There are several approaches to determine pore-water COPC concentrations:
- EqP to estimate pore-water concentrations from bulk sediment concentrations.
- Traditional sediment collection followed by centrifugation or core squeezing and filtration and/or flocculation of residual particulates. The resultant supernatant (pore water) can be chemically analyzed or used for toxicity testing.
- Sampling of pore water by use of suction devices, piezometers, or equipment such as the Trident probe. The resultant pore water can be chemically analyzed or used for toxicity testing.
- In situ or ex situ placement of diffusion or equilibrium-based samplers directly into the sediment or extracted pore water and measurement of either the sampler (e.g., SPME, PE, and POM) or the liquid contained therein (e.g., peepers).
Section 4.1.3.1 discusses EqP to estimate pore-water concentrations in detail. While pore-water concentrations of COPCs have shown to correlate with aquatic toxicity test results in relatively “clean” sediments, in sediments containing anthropogenic carbon (soot carbon, black carbon), estimates of pore-water concentrations from published EqP coefficients can overestimate pore-water concentrations by up to three orders of magnitude (Hawthorne, Grabanski, and Miller 2006). For this reason, other methods to measure pore-water concentrations of COPCs have been developed and are discussed below.
- easy to implement
- relatively low cost
- have a track record of field studies where the methods have been used
Disadvantages of Centrifugation Methods
- require a large amount of sediment to extract sufficient pore water for chemical analysis for some compounds (e.g., PCBs, PAHs) but not for others (e.g., metals)
- typically have high chemical detection limits due to the small volumes of pore water extracted
- disrupt the integrity of the interstitial pore space
- may create conditions (e.g., altered redox or pH) whereby pore-water chemical form or speciation are altered
Centrifugation methods are well documented (USEPA 2001c, NFESC 2003) and are arguably the simplest and most commonly employed method for extracting pore water. Various types of sediment core squeezing devices have been employed in the past to obtain pore water. Suction devices that employ a syringe that is joined (via tubing) to an air stone or sintered glass filter have also been employed. The groundwater sampling systems described previously are also being used more frequently at contaminated sites to collect pore water. The box at right lists advantages and disadvantages of these pore-water collection methods (see Appendix C‑T1).
In particular, the handling and disturbance of sediments that are reduced in situ can greatly increase the solubility of metals, resulting in false positive results. These methods are also not capable of completely removing COPCs associated with suspended particulates and DOC and thus do not report the truly bioavailable fraction of a COPC. A comparison of these types of pore-water collection methods can be found in Schults et al. (1992).
- can be done in situ or ex situ
- measure the truly dissolved (bioavailable) fraction in pore water
- may act as biomimetic or surrogate for benthic organism exposure for some COPCs
- can be used to monitor COPCs in coarse sediments or in sand caps that might not be amenable to sampling by conventional sediment sampling methods
- can be used to support TIE
In situ and ex situ pore-water measurements can be made using samplers that rely on selective diffusion over time (e.g., sediment “peepers”) or rely on a more rapid flux to attain equilibrium (e.g., POM, PED, SPMD, SPME). Diffusion samplers are routinely used for sampling HOCs. A number of the more commonly employed devices are described in Appendix C-T2. There are two general types of in situ samplers: passive diffusion samplers and equilibrium samplers. Some advantages of using these samplers are listed in the box at right. Diffusion samplers consist of a semipermeable membrane or dialysis tube filled with distilled water, a pure oil (triolein), or a gel, all of which rely on a diffusion gradient to establish equilibrium between the pore water and the sampler. Diffusion samplers are used for measuring metals, phosphates, and sulfides. Because diffusion samplers measure the bioaccessible (i.e., sediment extractable) fraction of COPCs, they require some knowledge of flux of a COPC across the membrane over time (e.g., by using internal permeability reference compounds) to correlate to biological uptake.
The pore-water sampler shown in Text Box 2-1 for the Mocks Pond site is one example of a passive diffusive sampling device. Another example is a sediment “peeper,” which typically employs a rigid body into which open cells are milled. The peeper is laid flat, the cells are filled with distilled water, and the cells are covered with a semipermeable membrane. A perforated acrylic cover holds the dialysis membrane in place but allows exposure of the cells to sediment (USEPA n.d. “Measurement”). Section 4.2.1.2 offers another example of a diffusion sampler where COPCs coming into contact with the sampler and having sufficient volatility (Henry’s law) partition out of the pore-water solution and diffuse across the semipermeable membrane to the adsorbent for collection.
SPMDs are long (~1 m), flat, low-density polyethylene tubes typically filled with purified lipid (triolein) and sealed. The flat SPMD is wrapped around a metal “spider” that holds it in place. Four spiders are then placed in a perforated stainless steel deployment device to provide protection when deployed into surface water or sediment (USEPA n.d. “Measurement”). SPMDs uptake contaminants until they are at equilibrium among the phases (typically water and polyethylene/lipid). The membrane mimics a biological membrane in its ability to allow selective diffusion of organic compounds from sediment or surface water (Zimmerman, Thurman, and Bastian 2000).
Diffusive gradient in thin films, another type of diffusion sampler, refers to two similar tools for collecting metals from sediment pore water (Davison et al. 2000). DGTs differ from other diffusive samplers in that they are typically casings filled with gels that are specific to the target compound (e.g., a Chelex or acrylamide gel for metals, ferrous-oxide gel for phosphorus). The unique advantage of DGTs over other diffusive samplers is that after retrieval, the gel can be cut into segments for multiple analyses.
Equilibrium samplers are often used for the measurement of pore-water concentrations of HOCs such as PCBs and PAHs. These types of samplers can be divided into those that are used to extract small quantities of COPCs from extracted pore water and those that are inserted directly into the sediment and accumulate HOCs in proportion to their presence (Fernandez et al. 2009). Equilibrium samplers may be used as biomimetic devices because they may mimic uptake from the solid phase or pore water directly to the organism (NRC 2003, Wenning et al. 2005).
Equilibrium samplers include SPME, PEDs, POM, and polydimethylsiloxane (PDMS) samplers that provide a measure of freely dissolved HOCs in sediment pore water. In many cases, the results obtained from the use of these samplers correlate with aquatic toxicity test results and/or bioaccumulation of the compounds into benthic organisms (Zimmerman, Thurman, and Bastian 2000; Van der Heijden and Jonker 2009; Hawthorne et al. 2007). SPME fibers have been used for both in-laboratory measurements of pore-water chemical concentrations and as a field-deployed method for measuring in situ pore-water concentrations of HOCs (Zeng, Tsukada, and Diehl 2004; Mayer et al. 2000; Reible et al. 2008, Hawthorne et al. 2007). To date, most of the in situ work has evaluated PAH bioavailability in sediments, where several researchers have shown that PAH uptake into the SPME fibers is related to PAH uptake by tissue in some organisms (Reible et al. 2008, Van der Heijden and Jonker 2009). More recently, PCBs have been examined, but the time needed to achieve equilibrium rates for specific congeners is still an area of active research (Reible et al. 2008).
One innovative method has been developed to directly measure pore-water PAHs at low detection limits (pg/mL) from sediment samples as small as 40 mL (USEPA SW-846 Method 8272/ASTM Standard D7363-07). The field-collected sample is transported back to the laboratory and centrifuged. Dissolved solids in the supernatant are flocculated, and the sample is recentrifuged to eliminate the particulate and dissolved carbon–bound COPCs. An SPME fiber is added to the supernatant to adsorb pore-water PAHs and is then injected into a gas chromatograph/mass spectrometer (GC/MS) for analysis. The pore-water concentrations measured by this method have been found to be reasonably good predictors of toxicity (survival) to Hyalella azteca, a sensitive organism for evaluating toxic PAH response to benthic invertebrates. This method provides a more exact characterization of PAH impacts than found through the use of bulk PAH concentrations and EqP to estimate pore-water concentrations (Moles, Holland, and Andersson 2006; Hawthorne et al. 2007).
PE and POM samplers are similar to SPMEs in their ability to sequester organic compounds from sediments. These samplers have the advantages that (1) they come to equilibrium rapidly, (2) molecular tracers can be added prior to deployment that allow for a direct measure of equilibration, and (3) high concentrations of HOCs can be concentrated during short field deployments (Fernandez et al. 2009, Tomasaewski and Luthy 2008). Uptake of PAHs and PCBs by benthic organisms has also been shown to be correlated to uptake by PE and POM samplers (Vinturella et al. 2004, Tomasaewski and Luthy 2008). PE samplers have been used as part of toxicity identification evaluations (Perron et al. 2009) and also have been shown to provide a useful tool to measure very low levels of truly dissolved PAHs in surface water (Moles, Holland, and Andersson 2006; Carls et al. 2004; Fernandez et al. 2009).
4.1.1.3 Surface-water sampling
Surface water is a route of exposure for many benthic invertebrate species and may require careful sampling and consideration of what measured fractions might be bioavailable. For example, many states characterize the health of streams, creeks, and rivers based on surveys of the “EPT” species: Ephemeroptora (mayflies), Plecoptera (stoneflies) and Trichoptera (caddisflies). These species can be exposed to COPCs in surface water via the external gills and the integument. In marine systems, clams, oysters, mussels, and other invertebrates pump water through their siphons, extracting food, oxygen, and COPCs.
Bioavailability in surface waters can be addressed by many of the same tools described for groundwater and pore water. Centrifugation, diffusion, and equilibrium samplers have all been used for assessing the bioavailable fraction of COPCs in surface waters (Burgess et al. 1993, Cornelissen et al. 2008). Sampling of surface water is well established in most states and in federal programs and will not be covered in detail here. Table 4-2 provides water quality sampling and characterization measures and considerations.
4.1.1.4 Tissue residue analyses
The most direct method for determining whether COPCs are available to organisms is to directly measure the internal chemical concentration (tissue residue) either in organisms harvested from the field or by placing clean organisms into the contaminated sediment and allowing exposure to occur (see Appendix C-T5). Tissue residue concentration measurements integrate chemical bioavailability, multiple routes of exposure, and assimilation into organism tissue. Tissue residue values can be compared to observed toxic effects in the field such as pathological lesions, tumors, or other subcellular effects. Databases of tissue residue toxicity data have been compiled and are publically available (USEPA MED database, www.epa.gov/med/Prods_Pubs/ecotox.htm; U.S. Army Corps of Engineers (USACE) WES database; Dillon and Gibson 1987; Beyer, Heinz, and Redmon-Norwood 1996; Jarvinen and Ankley 1999; Beyer and Meador 2011). Although these tissue residue toxic effects relationships can be used to estimate potential biological effects on benthic organisms based on tissue concentrations, it should be noted that the relationship between tissue residues and adverse effects is controversial, particularly for COPCs that are actively sequestered (e.g., metals via metalloproteins) or metabolized (e.g., PAHs). While measured tissue residues provide direct evidence that the chemical has indeed been accumulated, the disadvantage is that it is not necessarily possible to discern the route of exposure (e.g., sediments or surface water) and which is responsible for the adverse effects in the organism where multiple COPCs are measured.
Benthic organisms have been collected in situ as part of the Puget Sound Dredged Disposal Analysis Program, where the target species for measuring body burden are the sea cucumber Molpadia intermedia and the clam Compsomyax subdiaphana. Additional information on this study can be found at fortress.wa.gov/ecy/publications/summarypages/qa1.html (Washington Department of Natural Resources 2007).
For human health risk assessments (HHRAs), field collection of clams, oysters, and mussels and the subsequent measurement of tissue residues in the laboratory provide a direct measure of exposure concentrations to recreational and subsistence shellfish consumers (see Chapter 8).
Certain contaminants possess the ability to bioaccumulate, the process by which chemicals are taken up by an organism either directly from exposure to a contaminated medium or by consumption of food containing the chemical. As discussed earlier, benthic organism tissue residue concentration can be compared to observed toxic effects in the field such as pathological lesions, tumors, or other subcellular effects or to literature values/databases that contain tissue residue toxicity criteria.
When a site is determined to contain contaminants of concern (COCs) that bioaccumulate, it is important to consider the potential for biological transfer of these contaminants from benthic organisms to fish and wildlife (birds and mammals). The simplest method for estimating contaminant loads in biota is the use of accumulation factors (AFs), which consist of ratios of the concentration of a given contaminant in biota (e.g., fish tissue) to that in an abiotic medium (e.g., sediment). For the evaluation of sediments, this is commonly presented as the BSAF. The concentration in biota may be estimated by multiplying the sediment concentration by the BSAF (Bechtel Jacobs 1998a). It should be noted that AFs are generally used to assess risk to upper trophic level receptors and not the benthic organisms themselves. Therefore, a more detailed discussion of AFs and how they are used to evaluate risk to fish can be found in Section 5.2.3.1.
Additionally, the potential for adverse effects from exposure to bioaccumulative chemicals can be evaluated by using food-chain models to estimate dose(s). Dose estimates are then compared to receptor-specific food chain–based benchmarks such as no observable adverse effects levels (NOAELs), for example, ORNL Toxicological Benchmarks for Wildlife (Sample, Opresko, and Suter 1996).
4.1.2 Biological Methods
Methods for inferring bioavailability of COPCs to benthic organisms include bioassays (i.e., toxicity tests), benthic macroinvertebrate surveys, and bioaccumulation studies. Bioassays are often conducted to provide a more site-specific measure of sediment bioavailability after bulk sediment COPC concentrations have been shown to exceed SQGs. Site-specific benthic infaunal measures of abundance and diversity (i.e., macroinvertebrate surveys) can also be used to evaluate the overall quality of the macroinvertebrate community and may indirectly assess the bioavailability of site COPCs through the observation of effects (Chapman, Dexter, and Long 1987; Long and Morgan 1991).
Laboratory (e.g., bioassays with Lumbriculus or Nereis spp.) and field (e.g., caged organisms) bioaccumulation studies provide a means to assess uptake under controlled or more realistic conditions (Lee 1998; Lee et al. 1993, USEPA 1994c). See Section 4.1.1.4.
- Provide a measure of toxicity resulting from one or several chemicals at a site.
- Indirect measure of the bioavailable fraction of contaminants.
- Can be conducted using organisms, life stages, and physical conditions of interest a particular site.
- Standardized methods (ASTM, USEPA) provide a legal and scientific precedence for use.
- Are promulgated in several federal and state sediment management programs.
- Can include TIEs to assess COPC responsible for toxicity.
4.1.2.1 Toxicity testing
Standard toxicity tests do not distinguish the particular COPC(s) responsible for the observed toxic effect(s). However, if a toxic effect is observed but the COPC(s) is unknown, a sediment TIE may help determine which COPC(s) is responsible for the observed toxic response. Along with bulk sediment chemistry measurements, toxicity tests are required in many state and federal sediment testing programs. As indicated above, these types of tests should be used with caution where the tests may alter chemical conditions such as redox potential, which may be controlling bioavailability in situ.
Types of Toxicity Tests. Various types of sediment toxicity test methods are available (see Appendix C-T3). A few types are listed below:
- Bulk sediment toxicity tests involve the exposure of test organisms to sediments that may contain known or unknown quantities of COPCs. At the end of a specific exposure period, the response of the test organisms is examined in relation to a “measurement endpoint” (e.g., percent mortality, growth, reproduction, etc.). These metrics are then compared to the same metrics from a control and/or reference sediment material to determine relative differences.
- Interstitial pore-water toxicity tests expose aquatic organisms to pore water extracted from sediments. The use of this methodology is based on the assumptions that pore water is at equilibrium with the surrounding sediment, the water phase provides a direct route of exposure to infauna, and the bioavailable fraction in pore water is most responsible for observed toxicity. A positive bulk sediment test and a negative pore-water test (using the same sample and organism) could imply that direct ingestion of sediment is the cause of the toxicity (i.e., COPCs are mobilized in the gut of the organism). It is important to note that the use of aquatic species (e.g., Daphnia spp.) is conservative as these sensitive organisms would never be exposed to interstitial pore water.
- Elutriate exposures are typically used for evaluating “potential” toxicity associated with dredged sediment resuspension. Elutriate testing involves mixing test sediment with an aqueous solution and then analyzing the supernatant. Elutriate testing is well defined in the two USEPA/USACE dredged sediment testing manuals for inland and marine water disposal:
- Inland Disposal Testing Manual (USEPA 1998b) https://projects.itrcweb.org/contseds-bioavailability/References/Evaluation-Analytical-methods-USACE-EPA.pdf
- Ocean Disposal Testing Manual (USEPA 1991) https://projects.itrcweb.org/contseds-bioavailability/References/gbook.pdf
- Imply bioavailability but do not provide a measure of which COPC is responsible for the observed toxicity.
- Test results not always translated into chemical cleanup levels.
- Bioavailability can be altered by sample collection, handling, storage, and laboratory exposure.
- Test organisms may not represent indigenous benthic organisms at the site.
- Laboratory test results have inherent limitations in predicting field ecological effects.
In situ toxicity tests have been developed more recently to differentiate toxicity test responses in the laboratory from those conducted in the field. These tests use either surrogate organisms or indigenous species placed in chambers (cages, mesh bags, etc.). Since these chambers are placed in site sediment, organism exposure to sediments, pore water, and overlying waters is maximized. Compared with laboratory conditions, this type of test better represents real-world exposure (which may fluctuate dramatically), reduces sampling/experimental-related artifacts, integrates stressors over time, and allows for more natural interactions of potentially critical physical and chemical constituents. A disadvantage of this test is that impact to the test organisms from nonchemical stressors (e.g., low dissolved oxygen, redox, or high turbidity) are difficult to differentiate from COPC effects. These tests are also more expensive and time-consuming than laboratory toxicity tests.
Test Organisms. A variety of standard methods have been developed by the USEPA, ASTM, and some states. These tests include exposures to bacteria, algae, macrophytes, macroinvertebrates, and fish. The selection of the organism can have a major influence on the ecological relevance, success, and interpretation of the test results. Furthermore, no one test species is always most sensitive or best suited for all applications over the wide range of sediment characteristics. Some factors to consider in the selection of the test organism(s) include relative sensitivity to the COPC in sediment, biological relevance to the subject site, life cycle, degree of sediment contact, ability to culture/maintain the organism in the laboratory, and tolerance of a wide range of physicochemical conditions. Appendix C-T3 provides citations for the more commonly used bedded sediment, pore-water, and elutriate tests.
It is generally recommended that sediment toxicity tests incorporate two different types of test organisms and at least two measurement endpoints (survival, growth, and/or reproduction). Testing multiple species reduces uncertainty and limits the probability of false positive or false negative results. The importance of testing multiple species increases with the level of ecosystem protection desired and the need to define “significant” contamination in the “gray” zone (marginally contaminated sites) (USEPA 1994a). However, the use of one species with multiple measurement endpoints may be justified if sufficient research has been conducted on the particular COPC. Because the costs of sediment tests are generally equivalent between test species, the choice to reduce the number from two to one test organism can allow twice the number of samples to be tested.
Test Endpoints. The endpoints typically measured with sediment toxicity tests are acute (mortality) and chronic (growth, reproduction, behavior) endpoints. Acute tests can be used to demonstrate a significant exposure and effect at a site but cannot account for sublethal exposure and effects. Concentrations of COPCs in sediments may not cause mortality but may interfere with the ability of an organism to develop, grow, or reproduce. Chronic toxicity test exposures more closely approximate the types of endpoints of concern for organisms in natural environments and can be used to better evaluate potential impacts to benthic communities in moderately contaminated areas (USEPA 1994a). Many state and federal regulatory agencies consider chronic endpoints to be ecologically significant and recommend the use of chronic sediment toxicity tests.
Toxicity Test Results. In most sediment toxicity tests, the organism response to contaminated sediments is compared with the response observed in a both a clean control and a reference sediment. Various methods are available to determine whether a statistically significant difference exists when comparing the results of field samples to reference samples. A detailed description of these statistical methods is beyond the scope of this document. For additional information, please consult USEPA (2002e), CETIS (2006), and Environment Canada (2007).
Toxicity Identification Evaluations. As noted above, one of the disadvantages of standardized toxicity tests is the inability to identify the particular contaminant(s) responsible for eliciting the toxic response observed. This shortcoming makes it difficult to translate toxicity test results into the development of chemical-specific cleanup goals, particularly when sediments are known to contain multiple contaminant classes (PAHs, metals, PCBs, etc.). A methodology termed “toxicity identification evaluation” has been developed to aid in identifying the contaminant responsible for the toxic response. A TIE consists of an iterative series of tests in which various physical/chemical modifications are made to the contaminated sediment that eliminate the potential toxic effects of a class of chemicals. By conducting a series of these tests, the COPC(s) class can be identified. For example, the addition of a carbon source to sediments will tend to sequester HOCs, eliminating or severely reducing their impact on the test species. If the toxic response is not observed with the amended sediments, then the TIE is a good indicator that HOCs are the class of COCS. However, if the impact to the test organism is still present, then another modification is made to further classify the COPC. USEPA (2007b) provides further information on designing and conducting sediment TIEs.
TIEs can be conducted on interstitial water (i.e., pore water) as well as whole sediments. Prior to designing a TIE, a decision should be made as to what medium the TIE will test. The toxicity test results from the manipulated sediment or interstitial water tests are compared to baseline toxicity test results from a sample tested prior to sample manipulation to estimate effectiveness of each sample manipulation in removing toxicity. Removal of sample toxicity by specific manipulations aids in the identification of the probable compound class causing the toxic effect.
Interstitial water TIE methods have been developed to classify five groups of toxicants commonly found in sediments (USEPA 2007b):
- Aeration tests evaluate volatile or easily oxidized toxicants.
- Reverse-phase solid-phase chromatography identifies nonpolar organic toxicants.
- Addition of ethylenediaminetriacetic acid (EDTA) identifies cationic metals.
- Addition of Ulva lactuca test or zeolite identifies ammonia.
- Graduated pH manipulation identifies pH-dependent toxicants.
Whole-sediment methods have been developed to classify three groups of toxicants commonly found in sediments (USEPA 2007b):
- addition of Ulva lactuca (algae) or zeolite tests for ammonia
- addition of cation exchange resin and sulfide addition tests for cationic metals
- addition of Ambersorb, powdered coconut charcoal, or carbonaceous resin tests for nonionic organic chemicals
Macroinvertebrate Surveys
- Provide an in situ measure of the health of benthic community within an area of concern.
- Integrate interactions of multiple COPCs and not dependent on a single route of exposure.
- Readily related to ecosystem quality (e.g., quality of the prey base for higher trophic organisms that feed on benthos).
- Rapid bioassessment tools available for visual (qualitative) or metric (quantitative) evaluation of benthic integrity.
Disadvantages of
Macroinvertebrate Surveys
- Imply bioavailability, but do not provide a measure of which COPC(s) (are) responsible for the observed toxicity.
- Survey results are often confounded by variables not related to COPC toxicity (predation, seasonal differences, physical/ chemical sediment characteristics, food availability).
- Can be difficult to obtain statistical power.
4.1.2.2 Macroinvertebrate community surveys
Macroinvertebrates can be defined as invertebrates that are large enough to be seen by the unaided eye, can be retained by a U.S. Standard No. 30 sieve (0.5 mm), and live at least part of their life cycles in or on available substrate in a body of water. They are considered an important biotic component of most aquatic systems and play a significant role in the structure and function of ecosystems, including the processing and transfer of organic material and nutrient cycling. The macroinvertebrate community often represents most of the primary consumer biomass/production in an aquatic system food web, thus serving as the trophic base that supports upper trophic level species (e.g., fish, waterfowl, and other animals) (Thiel and Sauer 1999). Changes or shifts in macroinvertebrate community composition could have significant implications for higher trophic levels and energy flow pathways in the food web.
Assessments of benthic macroinvertebrate community structure and function are sometimes used to provide evidence of COPC-related effects in the environment and as such provide the third leg of the SQT. In addition, they are practical to assess for the following reasons:
- They are found in most aquatic habitats and are of a size that can be easily collected.
- They have limited mobility and are less able to avoid unfavorable environmental conditions.
- They can be sensitive to both short- and long-term changes in sediment and water quality.
- They can respond to a broad array of potential pollutants.
Methodology. As they relate to ecological assessments, macroinvertebrate surveys are typically conducted to determine whether the sediments at a given location(s) (USEPA 1990; Lazorchak, Klemm, and Peck 1998; Schmitt et al. 1999) are impaired (benthic community alteration) in comparison to a reference/control location (see Appendix C-T6). A survey consists of macroinvertebrate collection and sorting, organism identification, and data analysis. Data analysis often involves the generation of various metrics, including community, population, and functional parameters such as species richness and tolerance indices (Barbour et al. 1999). The metrics selected for use in a survey may be specified by the method or a state/ federal regulatory program. Some of the more common metrics used include total abundance, species or taxa richness, and percent contribution of dominant taxa. Metrics can be integrated simultaneously to derive an index which represents a score (generally a single number) reflecting the overall quality of the area studied. Various benthic indices have been developed in recent years to assess environmental conditions and benthic habitat quality in both fresh and saltwater ecosystems (see Appendix C-T3).
A detailed description of each component of a macroinvertebrate survey is beyond the scope of this document. Be aware that some states (e.g., Maine, Ohio) may have adopted state-specific guidance (Appendix A) tailored to unique habitats/program needs for how macroinvertebrate surveys are to be conducted.
Integration. The results of a well-conducted macroinvertebrate survey can provide direct evidence of impairment and an indirect implication of COPC bioavailability. Typically, macroinvertebrate survey results are used in conjunction with other tools, such as sediment chemistry and sediment toxicity tests (SQT), to provide a measure of ecosystem health. However, the results of a macroinvertebrate survey can also be used as a single line of evidence to determine environmental quality or the need for remediation.
Advanced Tools for Assessing Macroinvertebrate Health. An advanced reconnaissance tool for viewing benthic macroinvertebrate communities is sediment profile imaging (Text Box 4‑3). While unable to provide the same level of detail concerning species and numbers present, sufficient studies have been done to be able to demonstrate the link between observed sediment conditions and the quality of the overall benthic infaunal population.
SPI is an optical coring device that works like an upside-down periscope and takes cross-sectional images of the upper 20 cm of the seafloor. SPI has been used for more than 20 years as a tool for a number of benthic parameters, including sediment grain size, depth of the oxygenated sediments (apparent redox potential discontinuity), presence of methane gas bubbles, and placement of dredged or capped material. SPI has been used in dredged material characterization programs for the characterization of populations in contaminated sites, including the Hudson and Housatonic Rivers, a shipyard in San Diego, and at Soda Lake, Wyoming; in characterizing potentially deleterious organic debris at former log float areas or fish processing facilities in Washington and Alaska; and at numerous cap placement remedial programs, including the Eagle Harbor Superfund Site in Washington and the Permanent Shallow Water Habitat in Long Beach, California. Further information on SPI may be found at https://projects.itrcweb.org/contseds-bioavailability/References/bhmguide.pdf.

4.1.3 Predictive Methods
Predictive methods have been used to estimate the bioavailability of COPCs based on modeling or toxicity identification evaluations. These include the following:
- equilibrium partitioning
- hydrocarbon narcosis model
- accumulation factors
- simultaneously extracted metals–acid volatile sulfide
- biotic ligand model
4.1.3.1 Equilibrium partitioning
EqP is a widely applied model for estimating bioavailability and toxicity to infaunal organisms for nonionic organic compounds (e.g., PAHs, PCBs and pesticides). EqP assumes that equilibrium exists between the COPCs sorbed to the bulk sediment (OC) and the sediment pore water and that toxicity in sediments can be estimated by comparing the derived pore-water COPC concentration to effects concentrations previously measured in water-only exposures (i.e., NRWQC). Considerable evidence concludes that measures of pore-water COPC concentrations more accurately predict toxicity and observed community level effects than do whole-sediment concentrations (Di Toro et al. 1991, 2005a; Di Toro 2008; Hansen et al. 1996; USEPA 1994a, 2003d).
The prediction of the sediment concentration that causes toxicity is based on a single coefficient partitioning model that relates the toxic pore-water concentration to the equivalent sediment concentration (Di Toro 2008). In the EqP model, the observed variation in sediment toxicity is ascribed to the variations in the partitioning between pore water and sediment particles. More complicated models are possible that represent various types of organic matter (e.g., “black” or “soot” carbon) in sediment as well as the occurrence of dissolved OC in water; however, the EqP model results are easily compared to water-only effects concentrations and frequently provide a better indication of potential toxicity than the traditional approach of comparing bulk sediment concentrations to a sediment screening level concentration (i.e., sediment quality criteria [SQC]). Since pore-water concentrations derived from bulk sediment concentrations are easy to calculate, the comparison of derived pore-water concentrations to effect-level concentrations is often used as a second-tier analysis if bulk sediment concentrations exceed SQGs.
Nonionic COPCs are assumed to partition to bulk sediment OC. The pore-water concentration (Cpw) is predicted from the measured bulk sediment concentration (Csed) and TOC. In this regard, estimation of a site-specific Koc is beneficial in that it effectively incorporates the various organic matter phases present in the sediment as well as the site-specific influence of DOC levels. Values in the literature for the Koc of a specific chemical can vary due to the presence of multiple carbon phases. For example, Hawthorne et al. (2007) have shown that actual pore-water concentrations of PAHs can be overestimated by up to three orders of magnitude by using the EqP approach to derive pore-water concentrations. This error is due to the presence of anthropogenic carbon (e.g., soot or black carbon) in many sediments near urban and/or industrial systems, which have been shown to more tightly sequester HOCs than naturally occurring sediment OC. For this reason, USEPA (2003d, n.d. “Bioavailability”) has indicated that direct measures of pore-water PAHs are more accurate than derived pore water in sediments. Modifications to the EqP model have been made which include an anthropogenic carbon phase in addition to the fraction of naturally occurring OC (EPRI 2009). However, it has been shown that even this addition to the predictive EqP model may not provide accurate estimates of actual pore-water concentrations when compared to toxicity tests using Hyalella azteca (McDonough and Azzolina n.d.).
Equilibrium Partitioning Calculation Example:
Calculation of pore-water concentration from sediment concentration. EqP is used to calculate Cpw of nonpolar organic compounds based on Csed. The sediment-water partition coefficient (Kp) relates these two compartments through the following equation:
Cpw = Csed/Kp
The Kp value is derived from the compound’s Koc, which can be obtained from published sources (see USEPA’s EPI Suite quantitative structure activity relationship program, www.epa.gov/opptintr/exposure/pubs/episuite.htm) and the following USEPA ESB documents: PAHs—USEPA 2003d, nonionic organics—USEPA 2008b, and pesticides (dieldrin and endrin)—USEPA 2003c. In addition, Koc values can be derived from published octanol-water partition coefficients. The Kp value is the Koc adjusted by the fraction of organic carbon (foc) in the sediment:
Kp = Koc * foc
Calculation of potential toxic effect based on pore-water concentration. Pore-water concentrations are compared to water quality criteria to indicate whether they might pose a threat of impact to benthic invertebrates. The appropriate water quality criteria are final chronic values (FCVs), which are listed in the USEPA ESB documents (2003d, 2005c, and 2008b). The comparison of pore-water concentrations to the FCV is a ratio called a toxic unit (TU), where
TU = Cpw/FCV
TUs are calculated for each individual compound in the sample (Table 4-3) , and then these TUs are summed into a final TU value. A TU of <1 indicates no probable toxicity; a TU of >1 indicates potential toxicity. An example calculation for PAHs in a sediment sample containing 1% TOC (foc of 0.01) shows that all individual PAH TUs are <1; however, the summed TU is >1, indicating potential toxic effects.
Table 4-3. Calculation of potential toxic effect based on pore-water and sediment concentration
| Chemical | Csed (mg/kg) | Koc (L/kg) |
Kpa |
Cpwb (µg/L) |
FCV (µg/L) |
TUc |
| acenaphthene | .029 | 8.79E+03 | 8.79E+01 | 3.29 | 55.8 | 0.06 |
| acenaphthylene | 0.28 | 1.47E+03 | 1.47E+01 | 19.1 | 307 | 0.06 |
| anthracene | 1.75 | 2.87E+04 | 2.87E+02 | 6.11 | 20.7 | 0.29 |
| benz[a]anthracene | 2.88 | 3.77E+05 | 3.77E+03 | 0.76 | 2.23 | 0.34 |
| benzo[a]pyrene | 3.77 | 1.01E+06 | 1.01E+04 | 0.37 | 0.96 | 0.39 |
| benzo[b+k]fluoranthene | 5.58 | 1.49E+06 | 1.49E+04 | 0.37 | 0.65 | 0.58 |
| benzo[ghi]perylene | 2.55 | 2.49E+06 | 2.49E+04 | 0.10 | 0.44 | 0.23 |
| chrysene | 4.47 | 4.13E+05 | 4.13E+03 | 1.08 | 2.04 | 0.53 |
| dibenz[ah]anthracene | ND | ND | ND | ND | ND | ND |
| fluoranthene | 5.85 | 9.95E+04 | 9.95E+02 | 5.88 | 7.11 | 0.83 |
| fluorene | 0.34 | 1.37E+04 | 1.37E+02 | 2.52 | 39.3 | 0.06 |
| indeno[1,2,3-cd]pyrene | 3.61 | 4.06E+06 | 4.06E+04 | 0.09 | 0.27 | 0.32 |
| naphthalene | 0.20 | 1.99E+03 | 1.99E+01 | 9.83 | 193 | 0.05 |
| perylene | 1.42 | 1.07E+06 | 1.07E+04 | 0.13 | 0.90 | 0.15 |
| phenanthrene | 3.18 | 3.12E+04 | 3.12E+02 | 10.2 | 19.1 | 0.53 |
| pyrene | 5.20 | 6.90E+04 | 6.90E+02 | 7.55 | 10.1 | 0.75 |
| Sum | 41.38 | 5.18 | ||||
| a Kp = Koc * foc b Cpw = 1,000 * Csed/Kp c TU = Cpw/FCV |
The relationship between sediment, pore water, and biota is described schematically in Figure 4‑3. Note that the organisms are not assumed to be at equilibrium—the arrows to the biota are unidirectional, and the pore water and sediment particles are assumed to be at equilibrium. In addition, COPCs associated with suspended particulates or DOC are in equilibrium with the bulk-sediment and pore-water phases. The assumption for this model is that pore water is a good representation of the chemical activity of the compound in the system and therefore is assumed to be representative of all routes of exposure to benthic organisms. However, an important issue with deposit-feeding organisms is whether conditions in the gut of the organism modify the chemistry sufficiently so that ingested sediment cannot be assumed to be in equilibrium with pore water (COPCs may be more bioavailable from ingestion of sediment than from pore water).
4.1.3.2 Target lipid narcosis model
Narcotic chemicals are those that exhibit nonspecific effects on organism behavior (i.e., no target organ or specific site of toxicity is observed), and therefore their effects upon an organism are additive (USEPA 2003d). The target lipid narcosis model assumes that mortality will occur at a threshold level of a chemical in the organism’s lipid phase.
Narcosis theory is used to predict pore-water concentrations that cause acute toxicity to the organism from a particular chemical. While this is not a direct method for collecting pore-water data, it is a method used to evaluate pore water toxicity. The hydrocarbon narcosis model is one of the methods used to characterize sediment toxicity of nonionic organic compounds based on pore-water concentrations. The acute toxicity values are then converted to concentrations that are indicative of chronic toxicity, referred to as final chronic values. The FCVs are then compared to a particular site’s pore-water chemical concentrations to indicate the potential for toxicity, where
- pore-water concentration < FCV indicates no toxicity
- pore-water concentration > FCV indicates potential for toxic effect
The ratio of pore-water concentration to the FCV is termed a “toxicity unit.” Risk to the benthic community from narcotic chemicals can be evaluated using an additive TU approach (see Table 4-3 above). Narcotic chemicals often found in sediments include PAHs (USEPA 2003d) and 32 other nonionic organic compounds (USEPA 2008b).
4.1.3.3 ∑SEM – AVS
Bioavailability of some cationic metals in most anoxic sediments can be predicted by measuring the 1:1 relationship (in µmoles) between AVS and SEM (total SEM = sum of cadmium, copper, lead, nickel, silver and zinc). Both AVS and the sum of the SEMs (∑SEM) are liberated from wet sediment samples when treated with cold 1N HCl (hydrochloric) acid in the laboratory. The difference, termed “∑SEM – AVS,” is a useful tool for predicting metals bioavailability and toxicity (or lack thereof) to benthic organisms in sediments (Di Toro et al. 1990, Hansen et al. 1996, USEPA 2005c, Di Toro 2008).
Earlier literature cites the ratio of ∑SEM to AVS (i.e., ∑SEM/AVS). More recent literature, however, expresses the difference between ∑SEM and AVS (i.e., ∑SEM – AVS). The advantages to using ∑SEM – AVS are that it does not get very large when AVS is very low and that it can be modified to develop partitioning relationships that include other phases such as TOC (Di Toro et al. 2005a, 2005b).
The ∑SEM – AVS model is predicated on the same premise as the EqP model, i.e., the toxicity of metals in the sediment is directly related to its equilibrium between activity in sediment and the pore water. For cationic metals, however, solubility is theoretically governed by the strong complexation of cationic metals by sediment sulfides. By comparing the molar quantity of ∑SEM and AVS in a sediment sample, a measure of the bioavailable metal fraction can be estimated (Di Toro et al. 1990), where
- ∑SEM – AVS <1 indicates the ∑SEM are bound to sulfide (sulfide is in excess) and are therefore not bioavailable.
- ∑SEM – AVS >1 indicates the ∑SEM exceed acid soluble sulfide concentrations and therefore may be bioavailable.
Under the reducing conditions often found in sediments (typically higher in sulfate-rich brackish or marine waters), metals bioavailability is reduced as a result of precipitation of metals as insoluble sulfides (Text Box 4-4) because the solubility product constants for most metal-sulfide associations are very high and exchange from metal sulfides to water is low (NRC 2003, USEPA 2005c).
The calculation of (∑SEM – AVS)/foc is performed to determine whether potentially toxic divalent metals (cadmium, copper, lead, nickel, zinc and silver) are tightly sequestered by naturally occurring sulfides in surface sediment. An excess of AVS will ensure that the bioavailability of metals (and the probability for toxicity) is low; an excess of SEM may indicate the potential for toxicity, unless the sediment fraction of TOC is enough to act as another binding phase to bind metals that are not bound by AVS. The following data is from actual sediment samples obtained from the laboratory analysis of upper (0–3 feet) core samples in the Lower Hudson River (salinity ~10 ppt).
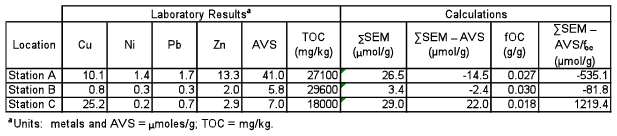
The laboratory results for individual metals required to calculate SEM, as well as AVS, are always reported as µmol/g of sediment (cadmium and silver were below the reporting limits for SEM). The results for TOC are reported as mg/kg.
The first step is to add all of the SEM metals to obtain a sum of the SEM (∑SEM). The result obtained for AVS is then subtracted from the ∑SEM. The next step is to divide the TOC result (reported as mg/kg) by 1,000,000 (mg/kg) to obtain foc (g/g). The ∑SEM – AVS difference is then divided by foc.
Per the USEPA ESB metals mixtures guidance (2005c), if the result is <130 µmol/goc, then toxicity to benthic invertebrates is not anticipated (Samples A and B). If the result is >3000 µmol/goc, then toxicity is likely (no samples exceeded this criterion in the above example). If the result is between 130 and 3000 µmol/goc, then toxicity is uncertain (Sample C).
The ∑SEM – AVS model is most useful in identifying conditions in which sediment toxicity is unlikely to occur. The ∑SEM – AVS paradigm has therefore been shown to be accurate predictors of the absence of mortality in sediment toxicity tests (Di Toro et al. 1990, Hansen et al. 1996, USEPA 2005c). At the time of the development of the ∑SEM – AVS paradigm, predictions of the actual toxicity in laboratory-spiked or field sediments were less accurate.
This uncertainty was later addressed by the valuable insight that the fraction of sediment organic carbon (foc) also plays a major role in the binding of excess divalent metals (Mahoney et al. 1996, USEPA 2005c). It was determined that when the ∑SEM – AVS was normalized by dividing by the foc, toxicity is likely when the (∑SEM – AVS)/foc is >3000 µmol/goc, uncertain when the concentration is 130–3000 µmol/goc, and not likely when the concentration is <130 µmol/goc (Figure 4-4).
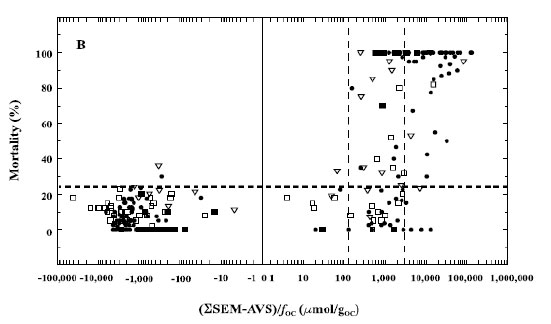
Figure 4-4. Relationship between (∑SEM – AVS)/foc and benthic mortality for both field and spiked sediments. (Source: USEPA 2005c)
A major uncertainty with ∑SEM – AVS is that even under reducing conditions, direct uptake of metal does occur in some infaunal species after ingestion of the various metal forms found in sediments, including metal sulfides (Luoma and Jenne 1977, Lee et al. 2000). The AVS method assumes no contribution to exposure from dietary metal uptake from either sediments or other food sources. Lee et al. (2000) showed that assimilation from diet was the best explanation for the poor correlation observed between measured cadmium, zinc, and nickel bioaccumulation by five different benthic species and the ∑SEM – AVS predictions (NRC 2003).
∑SEM – AVS also assumes that reducing conditions will be constant (i.e., reducing conditions at time of sampling must remain stable into the future). AVS, however, varies both spatially and seasonally. ∑SEM – AVS considerations are most applicable in sediment environments characterized by high levels of sulfate (e.g., estuarine or marine environs) and/or organic matter where bacterial activity can be expected to minimize oxygen penetration into the sediments, typically generating stable anoxic, reducing conditions (e.g., palustrine wetlands with seasonal die-off). The model does not account for potential dissociation during oxidation of the metal sulfide complexes (and thus increased bioavailability) that may occur with resuspension events nor the subsequent potential reformation of metal sulfides. An adequate modeling framework is needed that addresses the permanence issue, i.e., whether metals that are bound as metal sulfides can be considered to pose no reasonable risk even under changing conditions.
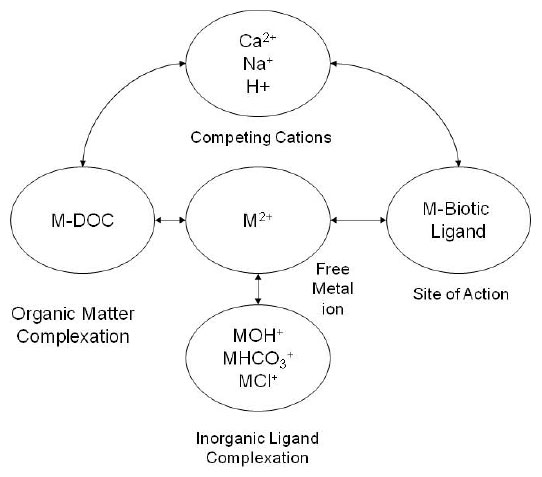
4.1.3.4 Biotic Ligand Model
The biotic ligand model (BLM, Figure 4-5) for cationic metals is also predicated on the same premise as the EqP and ∑SEM – AVS models for dissolved constituents (Di Toro et al. 2005b), in that the toxicity of a constituent in the sediment is directly related to the equilibrium concentration in the pore water. The BLM strictly addresses metals but involves an extensive set of equilibrium considerations to estimate the free metal (truly dissolved) concentration in pore water in relation to available biotic (and abiotic) ligands. Additionally, and this is an important distinction from other pore-water models, the model also incorporates biotic uptake as an equilibrium process and not a one-way exchange. Thus, ligand (absorbing) sites of an organism compete for the available free ion much as inorganic ligands (e.g., hydroxide or bicarbonate) and organic ligands (e.g., humic acids) do. Major and minor inorganic ligands may vary in importance from metal to metal. In other models, organic ligands in dissolved and sediment-bound forms are treated as a single organic matter ligand pool. Additionally, the model also deals with other (non-COPC) ions that compete for the biotic ligand sites (e.g., sodium and calcium), incorporating the parameter of hardness to compete with the uptake of the metal ion of concern.
These additions add a significant level of complexity to the model, as it is now necessary to approximate all dissolved forms of a metal as well as estimate the equilibrium “dissociation” constant for the biotic ligands. In some instances several ligand sites are suggested by the data, each with its own constant. Estimation of the biotic ligand equilibrium coefficient must also take into account the factors that affect inorganic and organic ligands, pH, ionic strength, temperature, and other solution-related parameters.
Recent work (Di Toro et al. 2005b, USEPA 2003a)suggests that a sediment-based BLM can be developed that will avoid some of the complexities in estimating the details of pore-water chemistry. Recent applications suggest that published results may be applied across multiple sites while biotic ligand equilibrium constants are developed for various species.
The development of the sediment BLM showed that the median lethal concentration on a sediment OC–normalized basis was essentially unchanged over a wide range of concentrations of pore-water hardness, salinity, DOC, and any other complexing or competing ligands. The sediment BLM showed that the most important factor affecting bioavailability of divalent cationic metals was the pore-water pH.
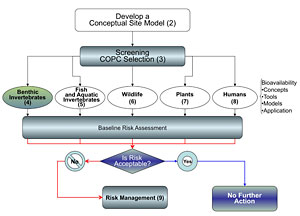
4.2 Application of Bioavailability Tools in Risk Assessment and Risk Management
This section is based on case study examples of how bioavailability tools and related measurements have been integrated into the sites’ risk assessment and risk management phases protecting benthic organisms. Although COPCs may not be bioavailable to benthic organisms in toxic amounts, this condition does not imply that they are not bioavailable and potentially transferable through the food chain. The potential for biomagnification to higher trophic level organisms should be identified in the CSM and planned for in the risk assessment. Assessment of bioavailability to higher trophic level organisms is covered in subsequent sections of this guidance. The tools described in this chapter identify the concentrations of COPCs that have the potential to move into the base of the food chain. These data can then be used for subsequent modeling to assess risk to upper trophic level receptors.
Stressor identification is the process of identifying the cause of an apparent toxicity response. Toxic responses in benthic invertebrate bioassessments may be caused by:
- Contaminants,
- Non-contaminant constituents (e.g. ammonia, sulfides), and
- Physical disturbances (e.g. low oxygen, hydraulic disturbance).
A process for identifying the source of toxicity could include:
- Statistical correlations between chemical sources of toxicity and results,
- Gradient analysis between chemical sources of toxicity and toxicity results,
- TIEs,
- Pore water measures, and
- Verification using spiked sediment bioassays and/or organism transplant.
At a minimum, bulk sediment chemistry analyses should be conducted at all sites. If sediment concentrations are above their respective screening values, then a second tier of sampling and analysis can be conducted, which can more specifically include bioavailability. These analyses may include site-specific chemistry endpoints (e.g., pore-water chemistry), sediment toxicity testing, and macroinvertebrate surveys. As mentioned previously, these three steps (chemistry, toxicity, and macroinvertebrate surveys) represent the Sediment Quality Triad (Long and Chapman 1985, Chapman 1996). It should be noted, however, that benthic macroinvertebrate surveys are highly dependent on the habitat conditions and may reflect differences in physical/chemical sediment properties that are not associated with the site-specific COPCs.
An example of a tiered approach is in the assessment of sediment PAH contamination. USEPA recently indicated that PAH effects to benthic organisms should be evaluated in the following tiered approach (Burgess 2009):
- Assess PAH bioavailability based on bulk sediment analysis (including comparisons to SQGs and the use of EqP to estimate pore-water concentrations for comparison to FCVs).
- Assess PAH bioavailability based on the analysis of interstitial water (i.e., direct measure of pore-water PAHs) and compare to FCVs).
- Assess PAH bioavailability based on aquatic toxicity testing (i.e., amphipod acute and chronic tests).
Successive tiers are evaluated only if the previous tier indicates a potential impact to benthos. In this case, successive tiers provide a higher level of certainty in the bioavailability analysis.
- Determine whether bulk sediment chemistry measures exceed SQGs or promulgated state standards.
- If these are exceeded, then compute COPC bioavailability using spreadsheet models of partitioning (EqP) or sequestration (SEM/AVS).
- If these are exceeded, then one or more of the following can be evaluated:
- pore-water chemistry using active or passive pore-water samplers
- laboratory sediment toxicity tests using site-appropriate organisms and conditions
- benthic macroinvertebrate surveys
- tissue analysis from field-collected organisms
- infer bioavailability in laboratory bioaccumulation exposures
As identified previously in this document, there are three principal areas where bioavailability data gathered during assessment activities can be used to make informed risk management decisions at a contaminated sediment site:
- risk assessment
- remedy selection
- remedial design/implementation/monitoring
- environmental dredging and monitoring
- monitored natural recovery
- long-term monitoring of cap performance
This guidance focuses on only the risk assessment phase; however, some of the case studies include discussion of bioavailability in the context of remedy selection and remedial design and implementation. A follow-on ITRC project and guidance will address remedy selection/design/implementation and monitoring.
4.2.1 Bioavailability in Risk Management
Risks are identified in the risk assessment based on exposure and effects assumptions. It is in the risk management stage where the decision maker must determine whether the information presented is sufficient to warrant an immediate remedial action or the overall evidence suggests that conditions exist that ameliorate the immediate concerns about risks. A good risk characterization articulates major assumptions and uncertainties, identifies reasonable alternative interpretations, and reaches scientific conclusions (USEPA 1998c). Bioavailability data can reduce uncertainty by providing more relevant information on exposure concentrations. This leads to a more realistic exposure assessment as compared to the conservative assumptions derived from bulk sediment chemistry alone.
Bioassessments are useful for identifying biological impairments, but they cannot identify the underlying causes of impairments. Stressor identification is needed to ensure that an impacted site is being cleaned up for the appropriate stressor (COPC) to protective levels. Other factors can influence toxicity tests or benthic macroinvertebrate community surveys. Nonpollutant constituents such as excessive detritus or organic materials, ammonia, phosphorous, sulfides, or microbial pathogens can directly influence toxicity tests and benthic community surveys. Chemical and physical factors such as changing salinity, low oxygen, very fine or coarse grain size, or hydraulic conditions (e.g., flood scouring or deposition, propeller wash) can also influence bioassessments.
USEPA (2000c) developed a general guidance document for the identification of stressors and has been the leader of development and use of TIEs in water and sediments. California has developed guidance for achieving SQOs in bays and estuaries that includes a description of a sequential series of actions initiated when SQOs are not met (California EPA 2009). These include stressor identification and use many of the tools to assess bioavailability previously discussed in this section.
In the case studies that follow, some or all of the stressor identification processes discussed previously were used to evaluate benthic exposure.
4.2.1.1 Vandenberg Air Force Base Site 5 Cluster (Bear Creek and Pond), California
The simplest example of toxicity source identification is conducting toxicity tests where bulk sediment measurements exceed the SQGs. At the Vandenberg site, the sediment investigation focused on a small freshwater creek and terminal pond that were contaminated with metals from former rocket-launching activities. The only risk pathway of concern was toxicity to benthic organisms. Bulk sediment chemistry analysis detected metals concentrations in sediment that approached or exceeded the SQGs. The California state regulatory agencies and the Air Force elected to conduct sediment toxicity tests with the amphipod Hyalella azteca to determine whether these metals were bioavailable at toxic levels. Sediment toxicity testing demonstrated that site sediments were not toxic, and as a result the site received a no further action (NFA) determination from the regulatory agency.
4.2.1.2 Lower Fox River, Wisconsin
The Lower Fox River and Green Bay Superfund Site in Wisconsin is principally a PCB-contaminated site, although other contaminants (e.g., PAHs, mercury, dioxin) were identified in the sediments of the river and bay. While multiple receptors (ecological and human) were evaluated, a comprehensive assessment of bioavailability to benthic organisms was conducted during the sediment remedial investigation.
For benthic infauna, calculated hazard quotients (HQs) based on PCB SQOs were high. Benthic infaunal community analyses showed that the system was largely dominated by pollution-tolerant oligochaetes and chironomids but that the system was recovering in place. Bioassays on bulk sediment samples collected from the same locations as benthic infaunal samples showed toxicity using the amphipod Hyalella azteca, the oligochaete Lumbriculus variegatus, the chironomid Chironomus riparius, and the mayfly Hexagenia limbata. Pore-water toxicity was also observed in acute and chronic bioassays on algae (Selenastrum capricornutum), invertebrates (Ceriodaphnia dubia), bacteria (Photobacterium phosphoreum), and fish (fathead minnow, Pimephales promelas). Measured body burdens in native infauna showed uptake of PCBs but not dioxins or PAHs. The results of the above studies would suggest that PCBs were impacting benthic resources. However, a TIE conducted on sediments from Operable Unit (OU) 4 and Green Bay demonstrated that ammonia, not PCBs, was responsible for most of the observed effects (Ankley, Katko, and Arthur 1990). The use of TIE testing determined that ammonia was most responsible for the benthic toxicity observed and that the PCBs did not play a major role in benthic toxicity.
This case study highlights how bioavailability testing, specifically using TIE, was helpful in that PCBs were identified as being “unavailable” to benthic organisms and were not the primary cause of benthic toxicity. Ultimately, this information factored into the USEPA record of decision (ROD) as the remediation for sediments was not based on the protection of the benthic community but rather on the protection of human health and upper trophic level receptors.
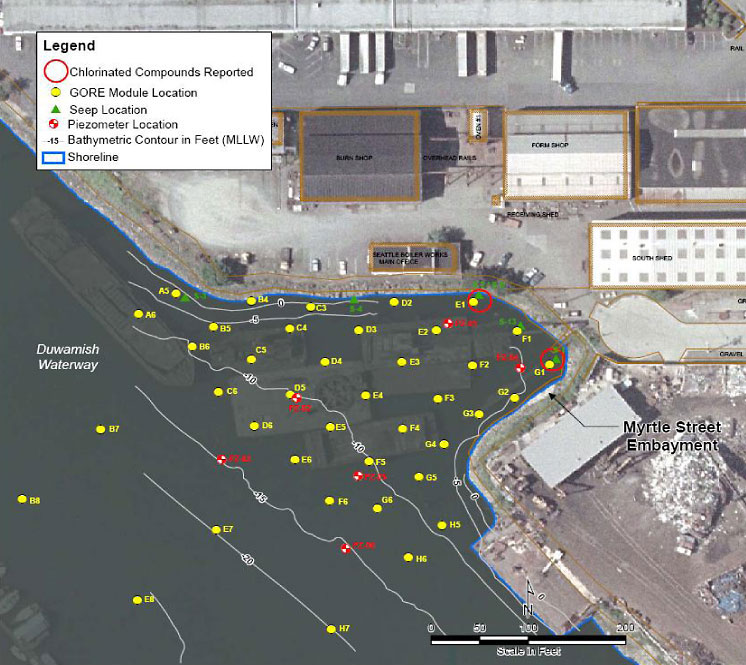
4.2.1.3 Myrtle Street Embayment, Lower Duwamish Waterway
The Lower Duwamish Waterway Superfund Site investigation focused principally on bedded-sediment contamination, but the site is also considered to be impacted by continuing releases to the system from surface water and subsurface groundwater discharges. Groundwater releases were principally evaluated by sampling seeps during low-tide sequences and by placing piezometers and peepers in the sediment. These approaches were not effective at all locations due to the need for rapid characterization over tidal cycles at a finer spatial grade. Peepers placed subtidally were also not thought to adequately capture volatile organic compounds (VOCs) at one particular site, the Myrtle Street Embayment Study Area.
Two overlapping discharging solvent plumes were identified at the Myrtle Street Embayment, a shallow aerobic plume that was mostly tetrachloroethene (PCE) and trichloroethene (TCE) and a deeper anaerobic plume of daughter compounds such as dichloroethene (DCE) and vinyl chloride (VC). Wells located 50 feet inland indicated that the plumes ranged from the top of the aquifer (at about 10 feet below ground surface [bgs]) to more than 45 feet bgs. The groundwater well concentrations were up to 1000 times groundwater cleanup levels while in seep samples concentrations were up to 100 times cleanup levels. However, as the freshwater plume encountered the tidal salt water wedge, the groundwater discharge area narrowed and rose over the wedge. In the process, the discharge area where benthic infauna would be exposed narrowed to about 10 vertical feet from 35 vertical feet. USEPA and the Washington Department of Ecology were concerned that the “worst” groundwater was discharging deeper in the waterway and therefore polluting the sediments.
Diver-placed diffusion samplers, GORE Modules were used to characterize the discharge to the embayment through localized seeps and generalized upwelling beneath the embayment. GORE Modules consist of GORE-TEX membrane tubes housing hydrophobic adsorbents which capture and measure VOCs and semivolatile organic compounds (SVOCs). These groundwater samplers (ITRC 2007, USEPA 2000b) were deployed in a systematic close-grid fashion across the embayment, known seeps, and potential critical discharge areas (Figure 4-6). Sampled over multiple tide cycles, the samplers were able to identify an expanded seep discharge face near the top of the saltwater wedge but demonstrated lack of a subtidal embayment discharge.
The results demonstrated there was no complete transition from groundwater to the bioactive layer of the sediment zone. Therefore, a complete exposure route did not exist between the contaminated groundwater seeping into the bay and the estuarian benthic community. The investigation showed an incomplete exposure pathway to subtidal organisms and, although a direct measure of bioavailability could not be discerned, the lack of detection of constituents of potential ecological concern (COPECs) by the GORE Modules did prove the null hypothesis, i.e., that potential receptors were not exposed and that there was therefore no risk to these receptors or their predators in the waterway. Without the investigation, the discharge area would have been assumed to be lower and more diffuse than it actually was, making it likely that additional biological sampling would have been placed in areas where exposure was limited.
4.2.1.4 Indian River Power Plant, Delaware
Cleanup levels based on the EqP-TU approach were calculated for intertidal sediments contaminated with NAPL and dissolved-phase diesel-range organics that resulted from a diesel fuel spill from a leaking underground pipeline into the Indian River sediments. The pipeline was taken out of service, and a sheet pile wall with sealed interlocks was installed to preclude the future migration of residual oil into the river sediments. Subsequent investigation work consisted of identifying the extent of impact, assessing risk to aquatic receptors, implementing a remedial action, and restoring the shoreline.
For each sample collected during the investigation of impact extent, bulk sediment chemical measures of PAH parent compounds and alkylated homologs were first normalized to the TOC concentrations at each corresponding sample point. Pore-water concentrations of these compounds were then predicted using EqP and were subsequently divided by analyte-specific acute and chronic values calculated from narcosis theory. For each sample, the TUs for individual compounds were summed to yield total acute and chronic TUs. TUs >1 indicated that pore-water exposure concentrations were potentially high enough to cause toxicity to benthic organisms. The state required excavation of all sediments with chronic TUs >1, which corresponded to a total PAH cleanup criterion of 2 mg/kg. In total, approximately 480 cubic yards of sediment was ultimately removed from the Indian River shoreline, and confirmatory samples indicated that the calculated cleanup criteria were met. Excavated sediments were replaced with clean material of similar grain size composition and were allowed to be naturally reworked and contoured over several tidal cycles prior to revegetation efforts.
A long-term monitoring program was subsequently established to ensure that the remedial efforts would remain protective of ecological receptors and included regular visual site inspections to monitor erosion and health of vegetation, photo-monitoring of vegetative growth and site development, vegetation sampling for various parameters, and sediment sampling for PAHs and TOC.
4.2.1.5 Onondaga Lake, New York
Site-specific remediation goals for the protection of benthic infauna were developed for the Onondaga Lake Superfund site. COPCs in lake sediments included benzene, toluene, ethylbenzene, xylenes, chlorinated benzenes, mercury, PAHs, PCBs, dioxin, and dibenzofurans. Separate preliminary remediation goals (PRGs) were developed for benthic, wildlife, and human health protection. Site-specific sediment effects concentrations (SECs) were developed for the protection of benthic infauna, as well as bioaccumulation-based sediment quality values (SQVs) for the protection of wildlife and preliminary remediation goals for fish. Tools employed to develop the SECs included bulk sediment chemistry, pore-water chemistry, toxicity testing, macroinvertebrate surveys, and tissue chemistry.
The sediment PRG was based on five site-specific SECs and one published PEC for the COPCs identified in the risk assessment. The SECs and PECs were calculated using data from paired sediment chemistry and acute sediment toxicity tests with Chironomus tentans and Hyalella azteca. Since the results for C. tentans were found to be the more sensitive test, these toxicity data were then used to develop the following five site-specific SECs: ER-L, TEL, ER-M, PEC, and AET. The geometric mean of these five Onondaga Lake SECs was calculated to provide a single consensus-based PEC for each COPC. For mercury, the PEC was calculated at 2.2 mg/kg.
4.2.1.6 Soda Lake, Wyoming
An NFA determination was reached for a former refinery evaporative pond at Soda Lake near Casper, Wyoming (see Text Box 4-5) From 1958 to 1990, Standard Oil built and operated a settling pond and evaporative basin to receive residuals from its oil refinery in Casper. After 1990, an estimated 1.7 million gallons per day (mgd) of water from the North Platte River were pumped to the lake to maintain the aquatic habitat for the over 300 species of birds, including 19 threatened and endangered (T&E) species that used the lake for migratory feeding and nesting. Other important species included red fox, wolverine, mink, prairie dog, and pronghorn antelope.
Soda Lake, Wyoming
COCs at Soda Lake at Soda Lake were principally PAHs, VOCs, metals, and selenium. After conduct of the RCRA facilitiesiInvestigation and corrective measures study, the WDEQ-selected remedy included closure of the Inlet Basin by removal of all contaminated sediments to a Corrective Action Management Unit, and an NFA for the Main Lake. The NFA was determined based upon extensive measurements that were used to evaluate bioavailability:
- bulk sediment chemistry
- pore-water chemistry
- groundwater flow and chemistry
- surface water chemistry
- bedded sediment toxicity testing
- EqP evaluations
- benthic and epibenthic surveys
- sediment profile imaging
- submerged or emergent aquatic vegetation
- benthic fish
- avifauna
- mammals

Subject to a Resource Conservation and Recovery Act (RCRA) facility investigation (RFI) under the direction of the Wyoming Department of Environmental Quality (WDEQ), the levels of VOCs, PAHs, and metals in the retention pond sludge were sufficiently elevated to warrant removal and disposal at a Corrective Action Management Unit. The RFI included ecological metrics at CSM-identified trophic levels, all of which contributed to the NFA for the evaporative basin. For benthic-related effects, the following measures contributed to the decision.
Triad Analysis. Triad sediment surveys suggested a potential impact from refinery residuals in the main lake. Sediment COPCs exceeded SQGs for PAHs, xylenes, phenol, and metals. Solid-phase bioassay testing was conducted to measure acute and subchronic toxicity of surface sediments. Low-level toxicity was observed for some stations, but toxicity was not correlated with chemical concentrations. In addition, when bioassays for some of the observed toxic stations were repeated, no toxicity was observed. Quantitative benthic surveys within the Main Lake showed low diversity but high abundance. An SPI survey confirmed the low diversity throughout the lake but also showed low dissolved oxygen levels. Qualitative assessments of epibenthic invertebrates showed a rich and diverse assemblage, including organisms that are important as prey for birds: insect larvae in the orders Odonata, Ephemeroptera, and Plecoptera, in addition to water fleas (Daphnia pulex), callenoid copepods, and amphipods (Hyalella azteca).
Bioavailability Assessment. COPCs measured in sediment pore water included acetone, phenol, carbon disulfide, and metals. Acetone was believed to be a laboratory artifact, and metals were shown to occur at background levels. Bulk sediment total PAHs were compared to total PAH threshold effect criteria (Swartz 1999), and no exceedances were observed. EqP was used to calculate maximum pore-water concentrations from observed bulk sediment concentrations. Even after applying a highly conservative 16-fold factor to account for unmeasured alkyl PAH compounds, predicted PAH TUs were <1.0. In surface water, organic COPCs were measured above their detection limits, but metals other than selenium were below NRWQC. Selenium levels were not an issue for benthic organisms but were addressed for higher trophic level receptors, including fish, birds, and mammals.
4.2.1.7 Tectronix Wetlands, Beaverton, Oregon
An NFA was determined for a reach of Beaverton Creek in Oregon based on results from bulk sediment chemistry, toxicity testing, and ∑SEM – AVS comparisons (see Appendix C). Historic operations at the Tektronix, Inc. site resulted in releases of metals that exceeded Oregon Department of Environmental Quality (ODEQ) Level II screening level values (SLVs) for freshwater sediments. Additional characterization of the sediments was conducted to assess the bioavailability of the sediment-associated metals and their toxicity to benthic organisms. Surface sediment samples were collected and analyzed for AVS, ∑SEM, total metals, grain size, total solids, and TOC. In addition, toxicity tests with Hyalella azteca and Chironomus dilutus were performed on a subset of surface sediment samples collected on site and at upstream reference locations. These locations were selected to provide good spatial coverage as well as a range of ∑SEM and AVS concentrations.
Toxicity testing showed that none of the sediment samples had an adverse effect on amphipods or midges based on the H. azteca mortality endpoint and C. dilutus growth endpoint. However, the results were somewhat confounded by the fact that increased C. dilutus mortality was observed in some samples when compared with one of the two upstream reference locations. The bioavailability of the sediment-associated metals and the likelihood that these chemicals would have caused the observed toxicity was assessed using ∑SEM – AVS. USEPA guidelines (USEPA 2005c) state that any sediment for which (∑SEM – AVS)/foc is < 3000 μmol/g OC poses an uncertain risk with regard to adverse biological effects resulting from metals toxicity. Sediment samples that showed an effect relative to the upstream station were less than half of that value. In addition, the USEPA guidelines state that the (∑SEM – AVS)/foc threshold above which adverse biological effects resulting from metals toxicity are expected is 3000 μmol/goc. The maximum (∑SEM – AVS)/foc result in any sediment sample was 360 μmol/goc, approximately ten-fold below the USEPA adverse effect threshold of 3000 μmol/goc. The assessment concluded that the surface sediment metals concentrations exceeding the ODEQ Level II SLVs were unlikely to be responsible for the observed C. dilutus mortality and that metals concentrations did not pose potential risks to the benthic community.
4.2.1.8 Hackensack River, New Jersey
Bioavailability of chromium in sediments in the Hackensack River near its confluence with Newark Bay, New Jersey (Magar et al. 2008, Martello et al. 2007) was evaluated as part of an assessment of risks and remedial alternatives. Chromium at the site was partly attributable to historical waterfront disposal of chromium ore processing residue. The key measures of bioavailability were presence and conditions that would favor formation of the toxic species hexavalent chromium (Cr6+) or the less toxic trivalent chromium (Cr3+) that is the prevalent form in reducing conditions commonly found in sediments. Measures and findings included the following:
- SPI surveys indicated that principally reducing conditions that favored formation of Cr3+ were found throughout the site except in a thin (<2 cm) surface layer.
- AVS measures across the site also confirmed the overall reducing conditions in the sediments.
- Pore-water sampling and analyses from the reduced sediments and within the top oxygenated layer never detected Cr6+; Cr3+ was found only at low concentrations in pore water despite bulk sediment concentrations as high as 2090 mg/kg.
- Cr6+ analyses in bulk sediment indicated detectable levels, but Cr6+ was not detected in a sediment resuspension and oxidation test following extended aeration and mixing with water.
- Biota tissue analyses showed no relationship between chromium concentrations in sediment and in tissue of laboratory-exposed and indigenous invertebrates. Concentrations in exposed organisms were within the range of those found in laboratory control organisms.
- Toxicity tests showed adverse effects of site sediments on amphipods but not polychaetes, even though the polychaete test species is known to be particularly sensitive to Cr6+. Effects on amphipods were determined to be associated with PAH, not Cr6+, concentrations. These findings were confirmed by tests at an upstream site affected by chromium ore processing residue that demonstrated no toxicity to amphipods at total chromium concentrations up to 1490 mg/kg (Becker et al. 2006).
Taken together, these and other lines of evidence demonstrated very low bioavailability of chromium in study area sediments and supported a decision of MNR.
4.2.1.9 Buffalo River, New York
At the Buffalo River Great Lakes area of concern, multiple bioavailability tools were used to develop of a site-specific remedial goal for total PAHs. The development of the remedial goal was based on USEPA’s Procedures for the Derivation of Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: PAH Mixtures (USEPA 2003d). Supporting lines of evidence included pore-water measurements of parent and alkylated PAHs, sediment toxicity tests using Hyalella azteca and Chironomus tentans, an evaluation of USEPA’s target lipid model, and bioaccumulation tests with Lumbriculus variegate. This case study focuses primarily on the use of pore-water analyses to measure PAH bioavailability and to establish site-specific EqP coefficients.
Pore water was collected Buffalo River sediment samples and analyzed for concentrations of 34 parent and alkylated PAHs using SPME followed by GC/MS. The pore-water results were combined with parent and alkylated PAH concentrations in whole-sediment samples and sediment TOC concentrations to determine site-specific, sediment Koc values for each measured parent and alkylated PAH. The Buffalo River Koc values are typically higher than USEPA’s default values (USEPA 2003d) and fall within the range of experimentally determined values from other contaminated sediment sites (Hawthorne et al. 2007). These results demonstrated that PAH bioavailability in Buffalo River sediments is less than predicted through models typically used to estimate chemical partitioning to sediments using default partition coefficients.
The site-specific partition coefficients calculated from the pore-water results were applied to the calculation of a site-specific PAH toxicity unit using USEPA’s EqP method. These results, along with additional lines of evidence, including the toxicity test results, were used to determine site-specific remedial criteria protective of benthic organisms and fish. Using default partitioning values would have resulted in an overly conservative remedial goal for PAHs, had site-specific partitioning data not been available.



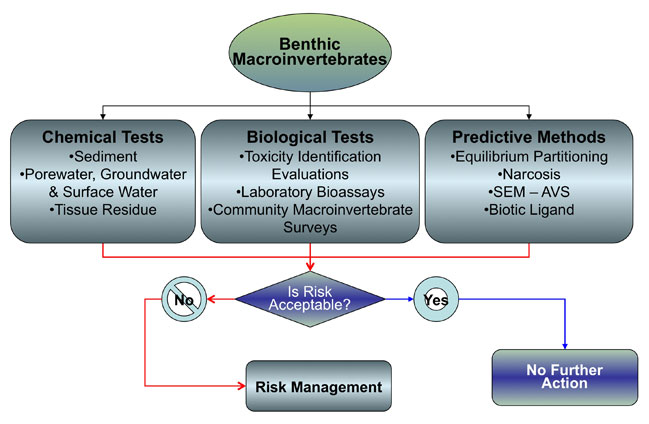 Figure 4-2. Tools and methods for evaluating benthic invertebrates for bioavailability.
Figure 4-2. Tools and methods for evaluating benthic invertebrates for bioavailability.