1.0 Introduction
As defined by the National Research Council (NRC 2003), “bioavailability processes” are the “…individual physical, chemical, and biological interactions that determine the exposure of plants and animals to chemicals associated with soils and sediments” (Figure 1-1). Specifically, bioavailability addresses the fact that only a fraction of a contaminant present in the environment may be taken up and subsequently result in an effect on an organism. Exposure of a chemical in soil, sediment, or pore water requires that the chemical come in contact with a biological membrane. The chemical can migrate through the membrane and enter the bloodstream, or a particle can come into contact with the membrane and the chemical can move from within the particle into the aqueous phase and subsequently move though the membrane to the blood (NEPI 2000). Based on this principle, the U.S. Environmental Protection Agency (USEPA) has defined “bioavailability” as the “state of being capable of being absorbed and available to interact with the metabolic processes of an organism” (USEPA 1992a), meaning that the chemical must (1) be released from the sediment (either in the natural environment [desorption] or after ingestion [bioaccessibility]), (2) come in contact with a membrane (e.g., stomach, intestine, lung, or skin), and (3) be distributed to an organ or cell. Bioavailability assessment tools aid in the assessment of human and ecological exposure and development of site-specific remedial action objectives (RAOs). An appropriate consideration of the degree of bioavailability, therefore, supports risk assessment and risk management decision making.
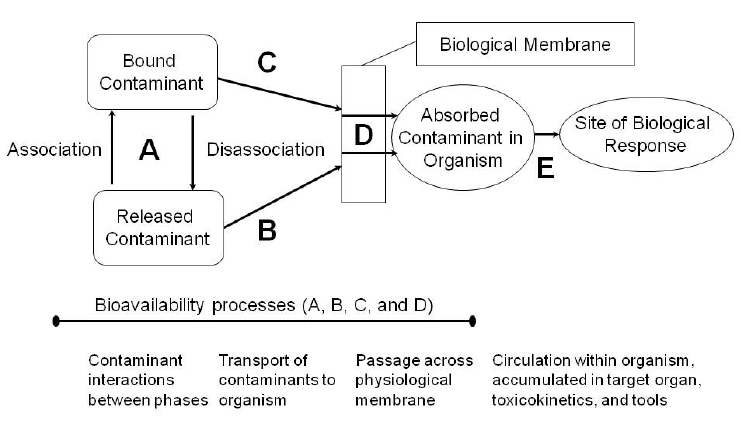
Figure 1-1. Bioavailability processes in soil or sediment. Includes release of a solid boundcontaminant and subsequent transport, direct contact of a bound contaminant, uptake by passage through a membrane, and transportation into a living system. A, B, and C can occur internal to an organism such as in the lumen of the gut. (Source: NRC 2003) .
This Web-based Interstate Technology & Regulatory Council (ITRC) technical and regulatory guidance on the use of bioavailability to evaluate exposure at contaminated freshwater or marine sediment sites describes the mechanisms affecting contaminant bioavailability, the tools used to assess bioavailability, proper application of those tools, and how bioavailability information is incorporated into risk management decisions at contaminated sediment sites. This guidance contains case studies presenting examples of the application of bioavailability in the establishment of remedial goals.
The ITRC Contaminated Sediments Team expects that this guidance will be used by responsible parties, state and federal regulators, practitioners, consultants, and public and tribal stakeholders, as a tool to understand how bioavailability can be useful in managing risk to ecological and human receptors at contaminated sediment sites. Because of the complexity of some of the material discussed in this document, the team assumes that the user has a reasonable understanding of the following:
- the risk assessment process
- contaminated sediments
- the potential value of using bioavailability assessment information
- basic knowledge of human health and ecological risk assessment terminology, methods, and approaches
The team recommends that, at a minimum, the user become familiar with USEPA ecological risk assessment guidelines (USEPA 1992b, 1997b, 1998c) before reading this document.
make a difference?
If contaminants are present but not bioavailable, they should not be included in the calculation of risk. This approach can optimize the extent of cleanup required to be protective and can be an important factor in balancing the risks caused by remedial action with the risks addressed by remedial action. This balance is particularly important for sediment sites where two of the primary remedial options, capping and dredging, can significantly alter physical, chemical, and biological conditions and disrupt or destroy existing habitat. At the Tektronix site in Oregon (see Appendix D), even though metals concentrations in stream sediment exceeded screening levels, a no further action determination was made based on evaluation of the bioavailability of the metals.
1.1 Background
State regulatory agencies are increasingly responsible for the identification, investigation, oversight, and management of contaminated sediment sites. The regulatory framework for management decisions for sediments is generally based upon USEPA’s human health and ecological risk assessment and remedial investigation (RI)/feasibility study (FS) guidance (Ehrlich 1988, USEPA 1989a, 1989d, 1992b, 1997b). States differ in the level of regulations or guidance for managing contaminated sediments. Currently, nine states (California, Florida, Minnesota, New Jersey, Ohio, Oregon, Texas, Washington, Wyoming) have developed detailed processes for assessing and designating sediments for management actions, while other states either rely on USEPA guidance or are developing specific guidance.
The current process used to assess sediment toxicity has included one or more elements of the Sediment Quality Triad (SQT, or “Sediment Triad”) approach (Long and Chapman 1985; Chapman, Dexter, and Long 1987; Chapman 1996; VonStackelberg, Thompson, and Patton 2008; Wenning et al. 2005). The Sediment Triad attempts to relate measures of bulk sediment chemistry, benthic community, and sediment bioassays to characterize contaminated sediments. Sediment quality guidelines (SQGs) evolved as an effort to identify thresholds for individual sediment chemicals that, when exceeded, adversely affect benthic communities and/or bioassay endpoints (Barrick et al. 1988; Chapman 1989; Chapman and Mann 1999; Long and Morgan 1991; MacDonald et al. 2003; Persaud, Jaagumagi, and Hayton, 1993; Barrick et al. 1988). Currently, SQGs are frequently used to determine the need for cleanup at many federal and state sites (VonStackelberg, Thompson, and Patton 2008). Additional discussion of these types of benchmarks appears in Chapter 3.
While the existing SQGs offer simplicity and utility (Wenning et al. 2005), those values are thresholds that focus only on benthic organisms. Unfortunately, SQGs generally do not address food-chain risks associated with bioaccumulation of sediment contaminants. SQGs often have low reliability or predictive value, and they are generally set on the conservative side (WDOE 2003) to ensure environmental protection. There is an increasing scientific and regulatory acknowledgement of the need to consider bioavailability processes of sediment contaminants in exposure assessments. Site-specific field measurements have clear scientific precedence over generic or literature-derived values. Based on sediment testing results for the Ashtabula Harbor site, MacDonald et al. (2005) found little site-specific evidence of PCB bioavailability or toxicity and much higher evidence of metals availability and toxicity, yet they dismissed the latter and concluded that the former drove toxicity and therefore management decisions based on generic literature-based “expected effects” concentrations, which their own data contravened at the particular site. Such a procedure weakens—in fact, practically eliminates—the technical credibility of the methodology in application. Ankley (1996), Di Toro et al. (2005a, 2005b), and Hawthorne et al. (2007) present evidence that identifies mechanisms that control contaminant bioavailability. The application of bioavailability in contaminated sediment management has lagged behind the still-growing body of evidence that confirms that at many sites sediment contaminants may be less “available” to cause harm to humans or ecological receptors than is suggested by extrapolating effects based on bulk (total) sediment concentration measurements (NRC 2003, SERDP and ESTCP 2008, USEPA 1998c).
An overwhelming body of scientific evidence points to the fact that physical, chemical, or biological properties can reduce the potential for sediment exposure and/or uptake of contaminants by living organisms. Processes affecting bioavailability are often not addressed when setting risk-based cleanup levels. Explicitly, assessing contaminant bioavailability can achieve more technically defensible cleanup goals and establish more accurate cleanup priorities while still ensuring protection of human health and the environment.
This document offers a compilation of the existing concepts, tools, and measures for assessing bioavailability. Case studies and examples of how those tools and measures have been used in decision making are also included. The ITRC Contaminated Sediments Team notes that the application of the tools described in this document may depend on a variety of project constraints, such as the following:
- schedule
- number of contaminants of potential concern (COPCs)
- investigation resources
- acceptance by the regulatory agency and regulated community
1.2 Objectives of the Document
The objectives of this guidance are as follows:
- provide a basic understanding of bioavailability
- provide direction as to where bioavailability considerations may be pertinent in the human health and ecological exposure assessment processes
- provide a direction to the pertinence of bioavailability during risk assessment process
- describe the tools of bioavailability assessment and their application
- describe how bioavailability considerations can be used in risk management of contaminated sediment sites
- provide case studies that highlight the application of bioavailability assessment tools and methodologies in contaminated sediment site risk management
This guidance assists state regulators and practitioners in understanding and incorporating fundamental concepts of bioavailability in contaminated sediment management, including communicating risk and the need for potential remedial action(s) to the public and other parties involved in the decision-making process.
An overview of the physical, geochemical, and biological mechanisms involved with assessing bioavailability is presented in Chapter 2. This technical and regulatory guidance does not provide a comprehensive description of those processes. For this level of detail, please refer NRC’s Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications (NRC 2003). Additional specific references are listed in Appendix A.
1.3 Sediment Assessment
Approach
Inclusion of bioavailability during a sediment assessment should proceed as part of the overall planning of a site investigation. Procedures for conducting a site investigation are described in several federal and state documents (see Appendix A). Those documents describe how to scope, plan, and execute sediment site investigations, risk assessments, and evaluate remedial actions. While there are differences in how each of those documents approaches site investigation, most generally adhere to the following four steps (see Figure 1-2):
- Development of a conceptual site model: Review site history, define initial COPCs, develop the conceptual site model (CSM), and identify initial RAOs and cleanup levels (Chapter 2).
- Screening-level assessment: Perform a screening-level evaluation that compares existing site sediment and/or fish/shellfish tissue concentrations to background and conservative screening criteria to finalize the COPC list and determine whether additional investigations or actions are needed (Chapter 3).
- Characterization of exposure and effect: Identify receptors of concern, assessment and/or measurement endpoints, exposure pathways, and data quality objectives (DQOs), refine nature and extent of COPCs, and conduct baseline human health and ecological risk assessments (Chapters 4, 5, 6, 7, and 8).
- Response action/risk management: Use the information gathered in the investigation and risk assessment stage to make an informed risk management decision and set defensible cleanup goals; identify and evaluate potential RAOs (Chapter 9).
While sites typically enter the assessment process at the scoping stage, they can exit at any point provided the following:
- the results indicate acceptable risk(s) to human health and ecological receptors
OR
- based on a risk assessment of all pathways, there has been a determination of unacceptable risk to human health and/or ecological receptors, but a site-specific remedial alternative is chosen that will manage or reduce risk(s) to acceptable levels
Incorporating bioavailability in the scoping process is an iterative process that is carried forward through each tier, as shown in Figure 1-2. For example, scoping activities are often revisited after completing a screening-level risk assessment as part of the planning for a remedial investigation and baseline risk assessment. Inclusion of bioavailability considerations as a project scoping activity allows for the evaluation of existing processes, available data, and the data needed for moving forward.
Figure 1-2 displays the general flow of an assessment of exposure at a contaminated sediment site. This flow diagram and related diagrams throughout this guidance help users identify where to consider bioavailability in the site investigation process and the tools and approaches that are available.The numbers in each box refer to the chapter of this document where the topic is discussed.In the diagram below, each box or circle with a number should be linked to the appropriate chapter identified in that box or circle.
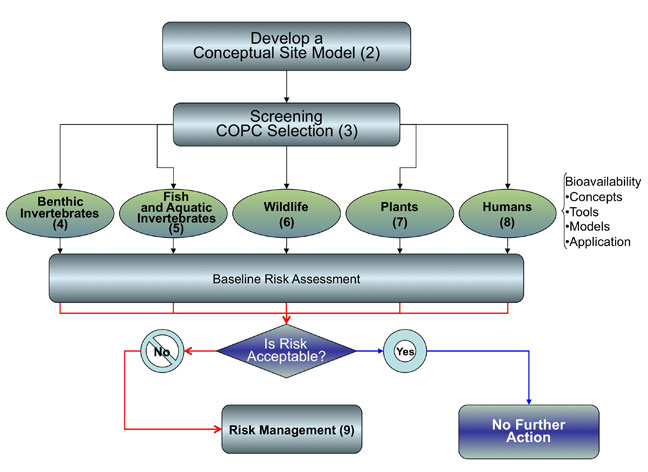 Figure 1-2. Sediment assessment process followed in this guidance.
Figure 1-2. Sediment assessment process followed in this guidance.This guidance provides a description of bioavailability considerations in the site investigation process. The following topics are described in each chapter of the document:
- Chapter 2 describes the process of scoping a contaminated site, characterizing the nature and extent of contamination, and risk assessment. The chapter identifies processes that can affect bioavailability in specific exposure pathways.
- Chapter 3 describes the use of background and screening criteria for ecological and/or human health. While traditional screening criteria have been developed empirically, more recent criteria are being developed using models that incorporate into estimates of endpoints bioavailability concepts other than toxicity, such as bioaccumulation.
- Chapters 4, 5, 6, 7, and 8 noted introduce and discuss the current in situ and ex situ measures and models used to assess bioavailability for specific exposure pathways. These tools assess exposures in sediments (e.g., benthic organisms) and the estimation of sediment-related contaminant transfer to water-column organisms (e.g., fish, amphibians), aquatic-dependent wildlife (piscivorous birds and mammals), and humans.
- Chapter 9 describes the role of bioavailability in risk management decisions, including examples of weight-of-evidence approaches, development of numeric cleanup criteria, and the state of the practice in evaluating long-term performance of bioavailability-assisted management actions..


