Technology Overview as part of a Web-based
Technical and Regulatory Guidance
Biochemical Reactors
1. Introduction
Click
Here to view case study table at the end of this document.
![]()
Biochemical
reactors (BCRs) treat mining-influenced water (MIW) by using microorganisms
to transform contaminants and to increase pH in the treated water. The most
commonly used BCRs for treating MIW are operated anaerobically (no oxygen)
and are also called “sulfate-reducing” bioreactors (SRBRs, SRBs). The microbial
process of sulfate reduction produces sulfide and bicarbonate within the
reactor, allowing the target metals such as cadmium, copper, nickel, lead,
and zinc in MIW to precipitate as metal sulfides at pH values above 5.0.
The bicarbonate promotes an increase in pH and will promote the removal of
some metals as carbonates such as FeCO3 and ZnCO3 under the appropriate conditions.
Conditions may include a specific pH and carbonate concentration. One of
the processes which occurs within the BCR is a change in the reduction-oxidation
(REDOX) potential of the MIW, and this aspect allows metals and metalloids
such as arsenic, chromium, selenium, and uranium to form precipitates. BCRs
may be applicable to the removal of a broad range of metal and metalloids
found in MIW. Conceptually, BCRs can be designed to address a wide range
of flows, acidity, and metals loading and also can be designed to operate
in gravity flow or available powered mode (Figure 1-1).
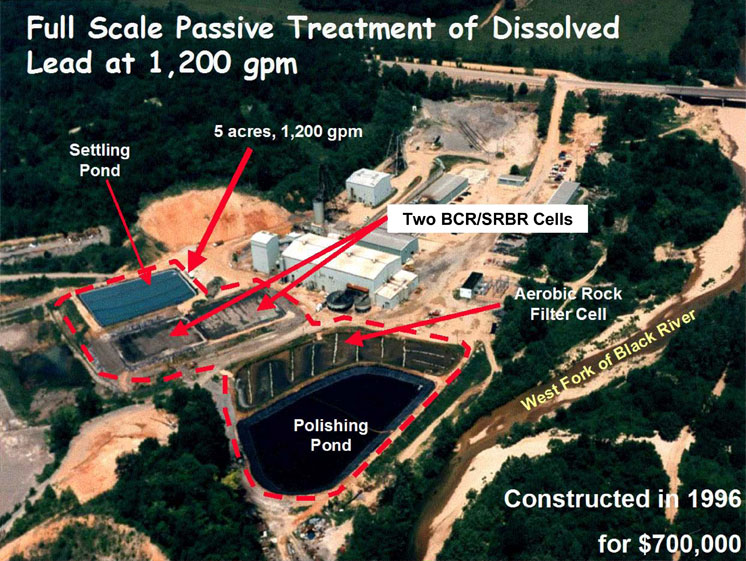
Figure 1-1. Example of passive BCR systems.
BCR design is affected by the kinetics of the desired microbial processes, which are strongly affected by pH and temperature, and also on the chemistry of the MIW. The chemically driven formation of metal precipitates is affected by the same factors. Thus, the design of BCRs is controlled by the site-specific MIW characteristics of pH, flow, temperature, and the type and concentration of metals. BCR design is also influenced greatly by available space, since the water to be treated must reside within the BCR for a certain period of time, called retention or residence time. In general, BCRs are at least 3–4 feet deep and may be deeper (6–8 feet) where freezing of the system may be a concern. The in-flow to the BCR is related to the residence time, and these factors influence the size of the reactor. As stated previously, most BCRs are anaerobic, but have related aerobic processes (see Constructed Treatment Wetlands Technology Overview). Finally, the in-flow to the BCR may be designed to enter either at the top of (downflow) or at the bottom (upflow) of the system.
In general, BCRs are broken into active systems, which require continuous energy and/or chemical input, and passive systems. The active systems may employ separate tanks or zones for the bioprocesses, chemical reactions, and solids separation. The potential exists for recovery of metals at active mining and mineral processing sites using a variation of the sulfate-reducing process. A lower operation and maintenance but active system has been tested at the Aspen seep at the Leviathan Mine superfund site. The system used an alcohol feed, rock support for the bacteria, sodium hydroxide for supplemental neutralization, settling ponds, and recycle pumping to optimize the process (USEPA 2006).
Most passive systems use designs that incorporate the bioprocesses, chemical reactions, and the bulk of solids separation within an organic substrate. These systems may also require pretreatment and polishing steps. Because the effluent of these anaerobic systems has low dissolved oxygen, the polishing step is an aerobic cell that increases the oxygen and also decreases the toxicity. The organic substrate is generally a mixture of locally available organic materials (e.g., wood chips, manure) and often contains limestone to provide additional neutralizing capacity and to increase substrate permeability. BCRs can be designed as open ponds (Gusek et al. 1998), buried ponds (Reisman et al. 2008), within tanks (Stowell Mine) or even in trenches between mine waste and the surface water body (Copper Basin).
2. Applicability
The following are applicable for the use of biochemical reactors:
- MIW
- acid to alkalinity pH
- wide range of metals and concentration
- wide range of flows
BCRs have been demonstrated to be effective in removing some metals from MIW, including cadmium, copper, cobalt, nickel, lead, and zinc. The primary target metals for sulfate-reducing BCRs are those that precipitate as a metal sulfide at pH values between 5 and 7. Additionally, some active metals and metalloids such as arsenic, chromium, selenium, thallium, and uranium may undergo a change in oxidation state, which is more amenable to precipitation in a neutral anaerobic environment. The size of the system is a function of metal loading rate, which includes factors such as flow, metals concentration, and required retention time. For example, a higher metal loading rate necessitates a larger system that requires more retention time and, in most cases, a lower flow rate.
BCRs are most effective in conditions where relatively steady flow rates and loading rates can be maintained and pH levels balanced to optimize overall treatment efficiency. Thus, flow equalization or pH adjustment may be required as a pretreatment step. The total metal loading rate is a function of flow, thereby affecting the required BCR system size. In addition, if the influent MIW pH is low (2–4), an increase in passive BCR area is required to accommodate the pH affect on the microbial community.
The chemical processes take time to occur within the reactor, and there is a minimum time for the MIW to be in the “reactive” part of the system. The contact time is typically called the “retention time,” and although the value will be reactor specific, it generally ranges 8–48 hours.
3.
Advantages
Active BCRs—
- can easily accommodate reasonable high flow rates
- easily integrate pH adjustment if needed
- can have reactive chamber or cell separate from other parts of the system
- can regulate flows, retention time, chemical addition, and pumping
Passive BCRs—
- have low operation and maintenance (O&M) requirements
- have low initial cost materials for construction
- use local materials for substrate
- have low technology construction practices
- can be constructed in remote areas
- can be sustained for months at a time with no human intervention
The use of active BCRs to treat MIW would allow for the treatment of relatively high flows in a small footprint. The use of active BCRs, with low energy and chemical input but which recycle (semipassively) like the rock-based system at the Leviathan Mine, is an attractive alternative. It should be noted that the amount of human intervention varies with the type of reactor. The Leviathan Mine uses some of the passive concepts but still requires 1–2 days a week for general O&M, BCR flushing every 1–4 months, and sludge collection from the ponds every 1–3 years. Low O&M time and cost do not mean an absence of O&M. The use of an active BCR allows various design configurations because the MIW may be piped into different chambers or the sulfate-reduction occurs in one cell while the precipitation occurs in another chamber. When it comes to sludge or precipitate removal, the main cell is not affected, so the system maintains its sulfide-generating capacity with little or no interruption in operation.
Passive BCR systems are attractive due to their ability to treat a wide range of MIW with the use of local materials and low O&M. Since the passive systems typically do not require any external power and can operate without continual maintenance, they are attractive for remote and abandoned sites.4. Limitations
- organic substrate longevity
- release of organics and nutrients from the BCR
- climate
- extremes in flow and influent chemistry
- permeability change
- space (footprint)
Organic substrates provided to the BCR may be readily or slowly soluble. Readily soluble substrates may include waste materials such as dairy whey or molasses or simple, well-defined compounds such as acetate, methanol, and ethanol. The advantages of alcohols are that they do not freeze and dosing can be regulated as a food source. The use of readily soluble substrates requires more O&M activities than systems with slowly soluble substrates alone. The use of complex substrates requires, and results, in a complex consortium of bacteria where fermenters produce the required food for SRB. Thus, the nuances of both populations may affect performance. With a continual source of organic carbon, reaction rates are much more consistent and will continue as long as the feed solution is supplied.
With a slowly soluble substrate reactor, all the organic material is supplied when the system is built. These systems require much less O&M than systems receiving readily soluble organics, but the reaction rate decreases over time as the solid organic material degrades. The design of the slowly soluble substrate mixture is important as materials used (e.g., hay, wood chips, mushroom compost) have several pools of carbon that biodegrade at different rates. The water-soluble fraction typically results in high activity over the first 6–9 months of operation and high concentrations of effluent organic carbon (Neculita, Zagury, and Bussière 2007). The remaining material produces a lower but sustained rate of sulfate reduction (Pulles and Heath 2009). Early designs incorporated the “rule of thumb” design value of 0.3 mole sulfate/m3/day (Gusek et al. 1998). There is a lack of data on the change in sulfate reduction over time, and estimates of substrate life have varied from around 5 to 30 years. The oldest man-made passive treatment system in the United States is approximately 14 years old.
Hybrid systems can be designed to provide higher levels of sustained sulfate reduction by careful selection of organic substrate and regular inputs of readily available organic carbon (Pulles and Heath 2009). Again, there is a trade-off between O&M requirements and achieving higher sulfate reduction rates.
4.2 Release of Organics and Nutrients from the BCRFor organic substrate systems, the initial effluent usually contains elevated biochemical oxygen demand (BOD), nutrients, and color. The increased release can last for several months, including a higher concentration of some metals as compared to the MIW influent. This increased concentration stems from particles that were adhering to the substrate used in the BCR construction. Steps to decrease this impact have included polishing ponds and changes in substrate. Often in areas with highly contaminated water and no aquatic life, the initial release is not a major concern.
The effluent of a BCR should be tested prior to release since the anaerobic reactor discharges water with low dissolved oxygen and some remaining metals. Neculita et al. (2008) tested a bench-scale system and found toxicity attributable to the iron; EPA has tested four field-scale pilots in 2009 and early results show a variation in toxicity, most likely attributable to low dissolved oxygen and sulfides (Butler and Reisman 2010).
4.3 Climate
The climate at potential BCR treatment sites can be a limiting factor.
Extended periods of severe cold can result in reduced performance, and
high flows can stress the treatment system. During winter, BCRs located
in cold climates may be less effective as a result of lowered microbial
activity. Insulating BCRs (e.g., layers of wood chips) can manage the
impact of seasonally low temperatures(Reisman et al. 2008). This may
require construction of larger and/or deeper BCRs to compensate for cold-weather
performance or where freezing occurs. Because the surface of uninsulated
BCRs may freeze, reactors built in the ground should be constructed with
all inlet/outlet structures and a portion of the reactor located below
the frost line. An important factor in cold-weather performance is the
temperature of the MIW. If the influent temperature is above 10°C, delivering
this water to a well-insulated SRB reactor has produced good winter operation.
Having an upflow system may also help to maintain the system near influent
temperatures. In areas with intense rainfall events or large spring flows,
equalization basins may be required for storage of larger volume flows.
4.4 Extremes
in Flow and Influent Chemistry
The pH range of sulfate-reducing bacteria
has been reported as 6 ≤ pH ≤ 9 (Widdell 1988), but the EPA pilot cells operated
below this range (Reisman et al. 2008). The typical pH effect on bacteria
is similar to a bell curve with a maximum rate and lower rates at pH values
on either side. Thus, lower pH would tend to produce low rates of sulfate
reduction. However, the ability of bacteria to acclimate and the existence
of acidophilic SRB may mitigate the overall effect. The pH is also important
in terms of chemical precipitation. For example, ZnS precipitates at pH 4,
while FeS does not. Low pH issues may be addressed by pretreatment and incorporation
of alkaline media within the BCR matrix. BCRs are not well-suited to
treat all MIW waters. Very low pH (<2) may adversely affect the microbial
consortium. Extremely high concentrations of certain metals can also
cause toxicity to the microbial community.
The negative effect of excess metals may result from the mass flux of dissolved metals exceeding the sulfide-generation capacity of the BCR, from inhibitory concentrations of a metal that reduces the rate of sulfide generation or metals that can precipitate as oxyhydroxides. For sulfate reduction to be successful in treating acid mine drainage, the rate of sulfate reduction must be equal to or greater than the rate of acidity input (metals + acid). If the rate of sulfate reduction exceeds the rate of acidity input, excess alkalinity is generated, and the pH of the drainage increases. If the sulfate reduction system were 100% effective at removing metals, then one mole of sulfate would be reduced for each mole of divalent metal precipitated. The removal of each mole of aluminum and ferric iron requires 1.5 moles of sulfate reduction (Dvorak et al. 1992, Eger 1992). Therefore, to remove all the metals and acid from the input, the required rate of sulfate reduction can be calculated from the following:
| Required rate of sulfate reduction = | Σ mmole divalent metals
(M+2) + 1.5 Σ mmole Al+3, Fe+3 + 0.5 (1000 x 10>-pH) |
The typical or “rule of thumb” design value that has been generally cited in the literature is 0.3 moles/m3/day. Therefore, the metal loading rate should be ≤0.3 moles/m3/day for metals to be precipitated as sulfides. Inhibitory metal concentrations for both sulfate-reducing bacteria and cellulolytic fermenters are of concern. Strategies to address the potential formation of iron and aluminum hydroxide precipitates include pretreatment, limiting influent iron and aluminum, designing for iron reduction, and increasing the BCR volume.
For the BCR to be successful, the system must be anaerobic. The concern with oxygen is that it is a more energetically favorable electron acceptor when compared to sulfate. The extent to which oxygen may affect a BCR depends on the total amount of oxygen input to the BCR relative to the sulfate-reducing capacity. If the oxygen flux is too high, the rate of sulfate reduction can be completely inhibited.
4.5 PermeabilityIt has been postulated that early prototype SRBs or BCRs typically failed after 2–5 years of operation due to loss of uniform permeability. This led to short circuits, preferential pathways for flow, or plugging (USEPA 2002). Short circuits and the development of preferential flow paths cause the treatment or reaction (retention) time to become too short and decrease performance in removing metals. Underlying causes may include collection of precipitated metal sulfides, collection of iron or aluminum hydroxides and gels, and, in the case of the original compost-based BCRs, a change over time in the physical properties, such as compression of the compost.
Corrective measures for compost-based BCRs include removal of the BCR contents and rebuilding. In the case of compost-free, rock-based BCRs, flushing circuits are designed and constructed into the BCR to clear accumulated material as part of the O&M of the BCR, More recent designs incorporate limestone, coarse wood chips, and limestone gravel to improve substrate permeability. Using a recycle system can avoid collection of precipitates within the BCR at the expense of increasing the level of O&M by adding a pumping system.
4.6 Available Space (Footprint)One of the reasons for the failure of passive systems is that the systems were not designed well. The space requirement for BCRs increases with a decreasing rate of sulfate reduction that can be achieved. The smallest footprint will result from feeding soluble substrate. The largest footprint will result from using a slow-release organic substrate. Additional factors that tend to increase the BCR volume and hence the footprint are low pH, high metals load, high metal concentrations, cold temperature, and decreasing permeability. In one example at an elevation over 11,000 feet, the reactor size was designed to incorporate several feet of wood chips for insulating the BCR and maintaining temperature for the reactor to operate year-round (Reisman et. al., 2008, 2009).
5. Performance
BCRs are effective in removing some toxic metals such as zinc, lead, and
copper from MIW, that easily precipitate as sulfides at pH between
5 and 7. Well-designed systems generally increase acidic pH to neutral values
and decrease metal concentrations by over 95% (Reisman et al. 2008, 2009).
Additionally, active metals and metalloids (e.g., arsenic, chromium, selenium,
and uranium) that form stable precipitates under neutral reducing conditions
may also be removed. Thus, BCRs may be applicable to a broad range of metal
and metalloids found in MIW. The case studies and references on bioreactor
systems show the potential for BCRs to treat a broad range of metals with
a range of hydraulic residence times. However, the bulk of the information
available on the performance of BCR’s is on bench- or pilot-scale systems
and tends to be several years’ duration.
The U.S. examples of long-term operation in a field-scale system are the West Fork Mine Site bioreactor system, which was designed to treat 1200 gpm and has been in operation since 1996 (Doshi 2006), and the Luttrell BCR near Helena, Montana (Reisman 2006). The Luttrell BCR is part of the EPA Ten Mile Creek Superfund site and treats leachate from an on-site repository. The Luttrell system, a joint project of EPA Engineering Technical Support Center and EPA Region 8, has operated since 2003, and data have been collected showing metals removal efficiency consistently above 95% for most metals. In 2008, after high-arsenic-laden material was added to the repository, the arsenic removal efficiency dropped considerably, most likely due to an overload to the BCR. The system has been handling 1–1.5 gpm but does not operate year-round. Plans are in development for plumbing improvements.
The lack of long-term performance data for full-scale systems is an issue. Projected long-term performance in terms of substrate replacement tends to be optimistic (20–30 years). The technology appears to provide significant overall metal removal in terms of percent, but consistently meeting National Pollutant Discharge Elimination System (NPDES) standards may be a problem. Site-specific conditions and regulatory requirements are key to determining the potential applicability of BCRs for a particular site.6. Costs
Bioreactors can be costly to construct since generally the systems are lined
and often contain additional components, such as settling ponds and/or aerobic
polishing cells. The West Fork system, which contained two parallel bioreactors
and an aerobic polishing cell, cost about $700,000 to construct in 1996 (Gusek
et al. 1998). This system was designed to treat a flow of 1200 gpm of circumneutral
mine drainage. In 2008, a similar system was designed for Soudan Underground
Mine State Park and included a settling pond, two parallel bioreactors, and
an aerobic polishing pond. The drainage was also circumneutral with an average
flow of 60 gpm and peak flow of 300 gpm. The low bid for the system was around
$600,000, but the system was not built due to concerns about the potential
to produce methyl mercury (Eger 2009). Bioreactors should have lower O&M
costs than standard chemical treatment approaches since they do not require
continuous inputs of energy and chemicals and can operate unattended for
extended periods of time.
There is a computer application for estimating abatement costs for treating MIW using both active and passive treatment methods. AMDTreat can assist a user in estimating costs to abate water pollution using a variety of passive and chemical treatment types. The current version of AMDTreat is v4.0 (https://amd.osmre.gov/).
7. Regulatory Considerations
Substrate longevity and the ability of BCRs to consistently meet stringent
NPDES standards are substantial regulatory issues. Depending on the quality
of the receiving streams, the initial release of organics and nutrients may
also need to be addressed. In some of the case studies, regulatory flexibility
was used to facilitate the use of BCR treatment. For example, numeric effluent
limits were negotiated at the Golinsky
Mine based on best management practices. The site is remote with access
only by ATV or boat.
8. Stakeholders Considerations
In addition to concerns about longevity and reliability of treatment, stakeholders
may have aesthetic concerns related to odor and water color. BCRs can produce
excess hydrogen sulfide, which smells like rotten eggs and is easily detected.
The initial effluent from the BCRs can be highly colored for about 3–6 months.
9. Lessons Learned
- Anaerobic systems using sulfate-reducing bacteria have successfully reduced sulfate with efficiencies that approached 100% for some metals.
- Alkalinity addition, generally in the form of limestone and occasionally sodium hydroxide, enhanced the activity of SRB. Though bacteria have been found to survive at pH 2.5, increasing the pH to at least 4.0 yields better performance. Most metal precipitation is more efficient at a higher pH.
- Low temperatures generally reduce SRB activity; however, installing biological components below grade, covering reactors with insulating material, or controlling winter inflows can minimize temperature effects. Subfreezing ambient temperatures do not preclude effective treatment.
- Sulfate-reducing bacteria sources may affect success of treatment, especially in very acidic conditions. Inoculating systems with tolerant sulfate-reducing bacteria previously exposed to AMD, or developing diverse, tolerant microbial communities through gradual exposure may improve treatment.
- Introducing excessive oxidized iron solids into bioreactors may negatively impact treatment systems. Positioning aerobic cells downgradient of bioreactors and allowing solids to settle out of bioreactor influent can help prevent this issue.
- Depletion of organic carbon in bioreactors creates a tendency for decreased performance with time. Laboratory and pilot tests of carbon sources, replaceable organic matter cartridges, and continuous liquid carbon source addition are strategies used to address this problem.
- Manganese is not effectively treated with SRBs.
- Engineering concerns include the following:
- Seasonally high flows commonly reduced system performance or caused overflow of untreated water. This can be remedied in some systems by having extra storage or treatment capacity and diverting clean water away from the system.
- Flow problems (hydraulic conductivity loss or preferential flow paths) are pervasive in constructed treatment wetlands, biochemical reactors, and permeable reactive barrier systems, due to precipitate buildup and substrate settling. Flushing systems, air injection, replacing reactive material, and using 70%–100% nondegradable matrix materials have all successfully restored flow.
- Recirculation can be used to moderate AMD acidity, increase treatment efficiency, and prevent clogging.
- Treatment effectiveness varies spatially and with flow variability, temperature gradients, and organic substrate variability. Increasing substrate uniformity enhances performance uniformity and overall performance.
- High BOD can pose water quality concerns when SRB-treated water is discharged; however, addition of an aerobic polishing pond or a rock-lined channel to retain organic matter and reoxygenate the effluent can reduce BOD. Nitrogen- and phosphorus-containing substrate may also elevate effluent concentrations and should be monitored and addressed if necessary.
- Bench- and pilot-scale tests are important to account for site-specific conditions in the design.
- There appears to be a trade-off between maintenance requirements and system performance. A truly passive system may be possible for small or less toxic AMD sources, but variation or high loading make maintenance necessary within the first decade of operation. Though all systems required less effort than daily operation of active treatment plants, maintenance requirements range from weekly to every few years to maintain performance.
- Treatment system design must incorporate considerations of AMD chemical composition, climate, space, cost, site accessibility, available materials, and discharge standards.
Table 1
Case Studies Using Biochemical
Reactors and Location
11. References
Butler, B., and D. Reisman. 2010. “Personal
Communication: Pre-Publication Draft of Metals Removal Efficiency and Ecotoxicological
Assessment of Field-Scale Passive Treatment Biochemical Reactors.” Cincinnati:
U.S. Environmental Protection Agency.
Doshi, S. M. 2006. Bioremediation of Acid Mine Drainage Using Sulfate-Reducing Bacteria. Prepared for U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response, Office of Superfund Remediation and Technology Innovation.
Dvorak, D. H., R. S. Hedin, H. M. Edenborn, and P. E. McIntire. 1992. “Treatment of Metal-Contaminated Water Using Bacterial Sulfate Reduction: Results from Pilot-Scale Reactors,” Biotechnology and Bioengineering 40: 609–16.
Eger, P. 1992. “The Use of Sulfate Reduction to Remove Metals from Acid Mine Drainage,” pp. 563–75 in Proceedings, American Society for Surface Mining and Reclamation Meeting, June 14–18, Duluth, Minn.
Eger, P. 2009. “Mine Water Treatment at Soudan State Park” in Proceedings, American Society for Surface Mining and Reclamation Meeting, May 30–June 5, Billings, Mont.
Gusek, J. J., T. R. Wildeman, A. Miller, and J. Fricke 1998. “The Challenges of Designing, Permitting, and Building a 1,200 GPM Passive Bioreactor for Metal Mine Drainage, West Fork Mine, MO,” presented at the 15th Annual Meeting, American Society of Surface Mining, Reclamation, May 17–21, St. Louis.
Neculita, C. M., B. Vigneault, G. Zagury, and B. Bussiere. 2008. “Toxicity and Metal Speciation in Mine Drainage Treated by Passive Bioreactors,.” Environmental Toxicology and Chemistry 27: 1659–67.
Neculita, C. M., G. Zagury, and B. Bussière. 2007. “Passive Treatment of Acid Mine Drainage in Bioreactors using Sulphate-Reducing Bacteria: Critical Review and Research Needs,” Journal of Environmental Quality 36: 1–16.
Pulles, W., and R. Heath. 2009. “The Evolution of Passive Mine Water Treatment Technology for Sulphate Removal,” presented at the International Mine Water Conference, Pretoria, South Africa. Water Institute of Southern Africa Mine Water Division and the International Mine Water Association.
Pulles, W., A. Van Mielcerk, A. Wood, and A. Batchelor. 2001. “Pilot-Scale Development of Integrated Passive Water Treatment Systems for Mine Effluent Systems.” WRC Report. No. 700/1/01.
Reisman, D. J. 2006. “A Systematic Approach for Mine Water Remediation by the U.S. EPA,” presented at Sustainable Modern Mining Applications, the 2006 EPA Hard Rock Mining Conference.
Reisman, D., T. Ruttcowski, J. Smart, and J. Gusek. 2008. “The Construction and Instrumentation of a Pilot Treatment System at the Standard Mine Superfund Site, Crested Butte, CO,” in New Opportunities to Apply Our Science, Proceedings, National Meeting of the American Society of Mining and Reclamation, June, Richmond, Va., R. I. Barnhisel, ed. Lexington, Ky.: American Society of Mining and Reclamation.
Reisman, D., T. Rutkowski, P. Smart, J. Gusek, and M. Sieczkowski. 2009. “Passive Treatment and Monitoring at the Standard Mine Superfund Site, Crested Butte, CO,” Proceedings, 26th Annual American Society of Mining and Reclamation Conference, R. I. Barnhisel, ed. Lexington, Ky.: American Society of Mining and Reclamation.
URS. 2003. “Passive and Semi-Active Treatment of Acid Rock Drainage from Metal Mines State of the Practice.” Prepared for U.S. Army Corps of Engineers.
USEPA (U.S. Environment Protection Agency). 2002. Anaerobic Compost Constructed Wetlands System (CWS) Technology. EPA/540/R-02/506. Cincinnati: National Risk Management Research Laboratory Innovative Technology Report.
USEPA. 2006. Compost-Free BCR Treatment of Acid Rock Drainage Leviathan Mine, California. EPA/540/R-06/009. Cincinnati: National Risk Management Research Laboratory Innovative Technology Report. https://www.epa.gov/aml/tech/leviathan.pdf
Widdell, F. 1998. “Microbiology and Ecology of Sulfate and Sulfur Reducing Bacteria,” in Biology of Anaerobic Microorganisms, G. F. Zehnder and J. B. Alexander, eds. New York: Wiley.
Other Resources
Béchard, G., H. Yamazaki, W. D. Gold, and P. Bedard.
1994. “Use of Cellulosic Substrates for the Microbial Treatment of Acid Mine
Drainage.” Journal
of Environmental Quality 23(1): 111–16.
Glombitza, F. 2001. “Treatment of Acid Lignite Mine Flooding Water by Means of Microbial Sulfate Reduction,” Waste Management 21: 197–203.
Gold, M. H., and M. Alic. 1993. “Molecular Biology of Lignin Degradation by Phanerochaete chrysoporium,” Microbiological Reviews 57(3): 605–22.
USEPA. 2001. “Sulfide-Containing Mining Wastes Technical Resource Document.” Unpublished draft. Cincinnati: National Risk Management Research Laboratory.
USEPA. 2003. SITE Technology Capsule for Passive Treatment Technologies for Metal Removal from Acid Mine Drainage at the Summitville Mine Site. EPA/540/R-02/507a. Cincinnati: National Risk Management Research Laboratory.
U.S. Office of Surface Mining. 2008. “AMDTreat Version 4.1c.” http://amdtreat.osmre.gov.
