Technology Overview as part of a Web-based
Technical and Regulatory Guidance
Passivation Technologies
1. Introduction
Click
Here to view case study table at the end of this document.
![]()
Passivation of acid-generating material involves oxidizing or protecting
the sulfide surface from water and oxygen. Preventing oxidation of sulfides
in situ by controlling the environmental conditions for oxidation is potentially
a viable alternative to treatment in perpetuity. The generation of acid mine
drainage can be likened to a chair, with the four components being the legs
(Figure 1-1). Remove any one component (or leg), and the stool does not stand.
As a result techniques for reducing metal sulfide oxidation involve removing
oxygen, water, bacteria, or the sulfide minerals.
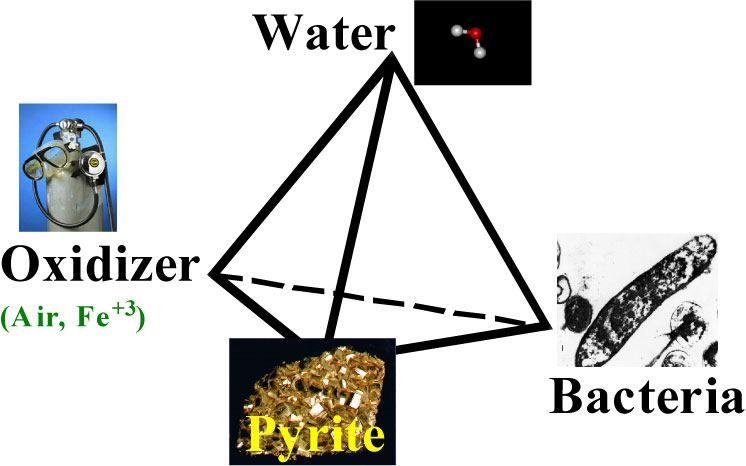
Figure 1-1. Acid rock drainage tetrahedron. (Courtesy Jim Gusek, Golder and Associates)
Since most mine waste already contains sulfides, techniques to coat and/or seal sulfide surfaces to reduce reactivity have been developed. Plastics, polymers, or cementation can be used to seal sulfidic surfaces (Moncrieff 2006). Surface passivation is analogous to galvanizing a nail; the outer layer resists oxidation. Passivated materials generally do not oxidize even if oxidation is an energetically preferential reaction. A variety of chemicals have been used in an attempt to passivate sulfide minerals; the most commonly tested include phosphate, silica, and permanganate.
Reactive mine wastes can be isolated from oxidizing agents (i.e., O2, Fe3+) by chemically precipitating a ferric coating on the surface of the waste material. This passivation process, sometimes called “microencapsulation,” prevents further oxidation of sulfide minerals by blocking the transport of oxidants to the sulfide surface and consuming ferric iron before it can become an oxidant.
The coating can be produced by reacting sulfidic material with low concentrations of an oxidizing agent in the presence of soluble phosphate or silica in a buffered solution. Hydrogen peroxide or calcium hypochlorite has been typically used as oxidizing agents. The oxidizing agent reacts with the sulfide to produce ferric ions:
FeS2 + 15/2H2O2 → Fe3+ +
2SO42–
+ 7H2O + H+ |
(1) | |
FeS2 + 15/4 Ca(OCl)2 + 1/2H2O → Fe3+ +
2SO42–
+ 15/4Ca2+ + 15/2Cl– + H+ (2) |
(2) |
Sodium acetate has been used to buffer the solution at a pH of 5 to 6. At this pH, dissolved ferric iron is unstable and precipitates as ferric hydroxide. If dissolved phosphate is present, it will scavenge ferric ions and ferric phosphate will precipitate:
Fe3+ + KH2PO4 → FePO4 + K+ + 2H+ |
(3) |
If silicic acid is present in the solution, it will react with the ferric hydroxides, producing an insoluble ferric silicate precipitate that is chemically stable at low pH (Evangelou 1996):
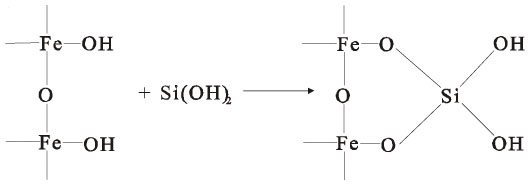
A number of studies have dealt with the feasibility of chemically producing coatings on reactive mine wastes and tailings. Reasonably successful coatings were reported in laboratory studies using phosphates (Evangelou 1994, Georgopoulou et al. 1995, Roy and Worral 1999), silicates (Zhang and Evangelou 1998; Fytas, Bousquet, and Evangelou 1999), and various organic materials (Adams, Ninesteel, and Rauch 1994; Moskalyk 1995). Generally, the presence of coatings on rock and tailing surfaces were confirmed using scanning electron microscopy (SEM), although decreased iron levels in drainage was often cited as evidence of coating formation.
Treatment of acid-generating rock with permanganate and magnesium oxides at a high pH (>12) is a patented process. Maintaining the high pH prevents the permanganate from disproportionating to a weaker oxidant and lowering Eh (De Vries 1996).
The DuPont passivation procedure uses the oxidizing ability of potassium permanganate to create an inert manganese-iron oxide on sulfidic rock. The sulfidic rock is treated with a permanganate and magnesium oxide slurry brought up to and held above pH 12. The layer has been shown to resist oxidation in oxidation simulation tests with hydrogen peroxide.
All passivated surfaces still have reactive rock below the surface, and oxidations will return once that passivation layer is removed. Thus, whether passivation is a viable option depends on time, other environmental conditions, and treatment efficiency requirement.
2. Applicability
- solid mining waste
- pit wall treatment
- surface applications
- solo technology or in conjunction with others
- multiple contaminants of concern
3. Advantages
- spray-on application
- source control
- may be long-lasting
4. Limitations
- not applicable for all cases
- treatment lifetime not known
- initial release of other constituents
- high cost
Since the chemicals are applied with water, the reactions and subsequent effectiveness are limited to the surfaces that can be contacted. This makes treatment at depth or in large stockpiles difficult, since the flow paths in mine waste are tortuous and complex.
Although the passivation compounds are predicted to be very stable, no long-term data are available to predict treatment lifetime.
A phosphate compound and a silica compound were tested for their ability to prevent acid generation and to evaluate application rates and treatment lifetime. Treatment with phosphate delayed but did not prevent oxidation, while the silica treatment has prevented acid generation for over six years (Eger and Antonson 2002, 2004; Eger and Mitchell 2007). With the phosphate treatment, there were elevated levels of phosphate and arsenic in the initial rinse waters. With the silica treatment, pH increased as the amount of treatment chemical increased, since the slurry contained lime in addition to the silica compound.5. Performance
The effectiveness of passivation chemicals to control release of acid and
metals was evaluated at two field sites: Gilt Edge Mine, S.D. (Final
Report: Remediation Technology Evaluation at the Gilt Edge Mine, South Dakota
Mine Waste Technology Program Activity 111, Project 29, 2005 and (https://www.epa.gov/region8/superfund/sd/giltedge/)
and the Golden Sunlight Mine, Whitehall, Montana (USEPA 2005). A subsequent,
larger-scale treatment was also done at the Golden Sunlight Mine. Most of
the discussion and results were taken directly from these reports. Each field
program is summarized below.
5.1 Gilt
Edge Demonstration
This project consisted of evaluating the use of silica
(KEECO), phosphate (EcoBond), Metals Treatment Technologies (MT2), and permanganate
(University of Nevada–Reno [UNR]) to stabilize acidic waste rock. Performance
was evaluated as a pilot- scale demonstration by placing treated waste rock
into isolated cells at the Gilt Edge Mine, monitoring the leachate collected
from the representative cells, and comparing the results to control cells and
to rock treated with lime. The leachate was monitored from the spring of 2001
to the fall of 2002. The objective of the treatments was to reduce the contaminants
of concern by at least 90% or to South Dakota water discharge limits. The three
technology vendors also provided a cost estimate to treat a hypothetical 500,000
yd3 waste rock pile at the Gilt Edge Mine using the pilot-scale data as a guideline.
By evaluating the leachate parameters of pH, total dissolved solids (TDS), dissolved arsenic, aluminum, iron, zinc, and sulfate, it was possible to ascertain if the technologies were able to achieve a 90% reduction or the South Dakota discharge limits. The results are summarized in Table 5-1 (USEPA 2004).
Table 5-1. Technology performance summary
Technology |
Achieve 90% reduction? |
Achieve South Dakota discharge
limits? |
Cost to treat 500,000 yd3 of
waste rock |
Comments |
|||||
Al |
Fe |
Sulfate |
pH |
TDS |
As |
Zn |
|||
Presumptive remedy, lime addition |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
$4,774,438 |
Effective, but pH was elevated above
8.8, and will fail once lime is exhausted. |
MT2 |
Yes |
Yes |
No |
Yes |
No |
No |
Yes |
$4,034,750 |
Increased TDS, sulfate, and arsenic concentrations. |
UNR |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
$3,241,408 |
Effective and has longer life than lime
treatment. |
KEECO |
No |
Yes |
No |
No |
No |
No |
No |
$12,682,998 |
Expensive and failed during second field
season. |
Based on the results in Table 5-1, the UNR and lime addition (presumptive remedy) technologies were able to achieve seven of the eight objectives. However, the lime treatment will be exhausted over time because the lime is soluble and will eventually dissolve.
The KEECO and MT2 technologies may be able to produce favorable results by making dosage adjustments and/or using different treatments; however, additional treatment past the second field season was beyond the scope of this technology demonstration. To confirm whether the modified KEECO and MT2 treatments would be effective, another technology demonstration would need to be performed.
5.2 Golden Sunlight Mine (GSM) DemonstrationThe intent of the demonstration project was to obtain performance data on the ability of four technologies to prevent the generation of acid mine drainage (AMD) from an open-pit highwall:
- EcoBond™ ARD (EcoBond) developed by MT2 of Denver, Colorado
- magnesium passivation technology (UNR/MgO) developed by the University of Nevada-Reno
- potassium permanganate technology (UNR/KP) developed and patented by DuPont Technology with field applications developed and applied by UNR (the current patent holder)
- a furfuryl alcohol resin sealant (FARS) developed by Intermountain Polymers of Idaho Falls, Idaho
The demonstration was conducted at GSM in an active open-pit gold mine located near Whitehall, Montana. The four technology providers spray-applied their technologies, which were in a liquid form, to a designated 50-foot-high by 50-foot-wide area on the highwall. A background/control plot of the same size was designated and used to evaluate and compare to the four treatment technologies. To evaluate and determine whether the objectives had been achieved, two test procedures were used: humidity cell (HC) testing in the laboratory and a field mine wall sampling method.
Data from the untreated GSM highwall, for both field monitoring and HC laboratory testing, showed that untreated material would produce acid in a natural weathering and oxidizing environment. The same background data from the untreated GSM plot were used for comparison of all the treatment technologies to determine if the technologies were effective in reducing the potential for AMD.
Humidity Cell (HC)Tests
During application of the technologies, each technology provider was required
to apply the technology to a specially prepared sample that was sent to McClelland
Laboratories, Inc. (MLI) in Sparks, Nevada, where American Society of Testing
Materials (ASTM) D5744-96 for Accelerated Weathering of Solid Materials using
a Modified HC testing method was conducted. For the HC testing, the technology
was allowed to contact the full surface area of the sample being treated
for an extended period of time, allowing for the most ideal application conditions.
The HC testing results were used to predict whether the untreated and treated
samples would produce acid and mobilize metals.
Results from 41 weeks of HC testing indicated and predicted that all technologies were effective in preventing acid production and the mobility of metals. Each technology was compared with the background sample results.
When compared to the background plot for EcoBond technology, the pH was neutral; the electric conductivity was typical for systems exposed to air and indicated minimal metal mobility; iron (Fe), sulfate (SO4), and acidity production were higher; and calculated ratios were substantially greater than regulatory guidelines. For the two UNR technologies, the pH was slightly greater than 6; the electrical conductivity was typical for systems exposed to air and indicated minimal metal mobility; Fe, SO4, and acidity production was higher; and calculated ratios were substantially greater than regulatory guidelines. Essentially, no metals were mobilized from EcoBond, UNR/MgO, and UNR/KP cells. The lack of metals mobility indicates that three treatment technologies prevented acid production.
For the FARS technology, the pH ranged between 4 and 5, the electrical conductivity was typical of systems exposed to air and indicated some metals mobility for Fe and SO4. The FARS-treated sample did prevent AMD but not as well as the other three technologies. Because the FARS technology has binding/stabilizing capabilities, the FARS HC sample had to be broken apart to allow it to fit into the HC test cells, which exposed rock surfaces that otherwise would have been covered.
Residual Wash Sampling Tests
After the technologies were applied to the GSM highwall, a mine wall/residual
wash water sampling test method that was developed for the Canadian Mine
Environment Neutral Drainage Program was implemented. The sampling test included
information where the total metals loading per unit area and the pH of the
highwall in the field were calculated and measured, respectively. This method
allowed the technologies to be evaluated under field conditions and field-designed
application rates.
Field results for the mine wall sampling show that for the EcoBond, UNR/MgO, and UNR/KP plots, the pH was as low as the pH of the background plot. This means that the pH was <4 and the range of average percent metals reduction was between -211% and 82% (see Table 5-2). The FARS recorded pH was steady at pH 4–4.5, extending for the full demonstration, and the percent metals reduction ranged 75%–91% compared to the background results.
Table 5-2. Percent reduction of total metals from the treated technology plots compared to the untreated plot (Plot A)
Metal |
FARS |
EcoBond |
UNR/MgO |
UNR/KP |
| A1 | 75 |
20 |
38 |
62 |
| Cu | 85 |
-211 |
26 |
76 |
| Fe | 85 |
24 |
-16 |
30 |
| Mn | 84 |
49 |
82 |
51 |
| Ni | 90 |
48 |
50 |
72 |
| Zn | 91 |
-40 |
75 |
76 |
Note: A large negative number for the percent metals reduction indicates high metals mobility, and a high positive number indicates a low mobility. |
In the field, physical stabilization of the highwall was observed on only
the FARS technology plot. The other three technologies provided chemical
passivation of the wall but not physical stabilization.
6. Costs
Theoretical costs for highwall treatments varied from $6/yd3 for the permanganate
to over $24/yd3 for the silica treatment (Gilt Edge demonstration project).
In a comparison of FARS, UNR/MgO, UNR/KP, and EcoBond processes, costs were
broken down to include materials, installation, and oversight. Mobilization
and shipping costs appear to be primarily dependent on distance and not on
the special equipment or other needs. The core cost elements are listed in
Table 6-1. Unit costs varied $2–$8 per square foot.
Table 6-1. Costs to treat highwall, Golden Sunlight technology demonstration
| Technology | Material |
% of total |
Install |
% of total |
Oversight |
% of total |
Total |
Unit cost* |
| FARS | $3,600 |
68% |
$1,695 |
32% |
(1) |
0% |
$5,295 |
$2.12 |
| UNR/MgO | $3,780 |
41% |
$2,948 |
33% |
$2,394 |
26% |
$9,155 |
$3.65 |
| UNR/KP | $3,780 |
41% |
$2,948 |
33% |
$2,394 |
26% |
$9,122 |
$3.65 |
| EcoBond | $10,250 |
54% |
$5,884 |
31% |
$2,910 |
15% |
$19,044 |
$7.63 |
| * Basis, 2,500 ft2 (1) Oversight assumed to be part of the installation cost. |
||||||||
7. Regulatory
Considerations
Passivation is a new technology, and the lack of large-scale, long-term data
creates regulatory barriers. Although some of the initial data suggest that
treatment can be effective, there are very limited data on long-term performance.
In addition, several studies have indicated that there is an initial release
of other constituents that would need to be evaluated. Techniques to control
and possibly treat this release may be needed and regulatory approval obtained
in advance of any release of these constituents.
8. Stakeholder Considerations
Since this is a new technique with limited data, stakeholders are likely
to be wary of its applicability and effectiveness.
9. Lessons Learned
9.1 Stockpile Treatment
The effectiveness of passivation technology is a function
of the contact of the treatment chemicals with the sulfide mineral surfaces.
Application to existing waste rock stockpiles is unlikely to be successful
without moving the material and treating it in smaller batches or lifts.
The actual dose of the chemical is an important variable affecting both
performance and cost.
9.2 Highwall Treatment
Problems were encountered
in the experimental program which affected the evaluation of the technologies.
Several mine wall sampling ports were lost when mine wall movement caused
the highwall to become unstable. The loss of the sampling ports had the
potential to affect the overall results. Due to the instability of most
highwalls, additional sampling ports should be included. In addition, there
was a possibility that airborne particulates and runoff from untreated
areas affected the field results. This needs to be considered in future
applications.
Table 10-1. Case study used in this technology overview
11. References
American
Society for Testing and Materials. 1996. Standard Test Method for
Accelerated Weathering of Solid Material Using a Modified Humidity Cell.
D5744-96 ASTM. West Conshohocken, Pa.
Adams, R. L., J. J Ninesteel, and H. W. Rauch. 1994. “Laboratory Testing of Coatings for Prevention of Acid Drainage in Underground Coal Mines,”. pp. 218–25 in Proceedings, International Land Reclamation and Mine Drainage Conference and 3rd International Conference on the Abatement of Acidic Drainage, April 24–29, Pittsburgh.
De Vries, N. H. C. 1996. “Process for Treating Iron-Containing Sulfide Rocks and Ores.” U.S. Patent No. 5587001. www.patents.com/us-5587001.html.
Eger, P., and D. Antonson. 2002. “Use of Microencapsulation to Prevent Acid Rock Drainage,” report to MSE Technology Applications, Minnesota Department of Natural Resources, St. Paul, Minn.
Eger, P., and D. Antonson. 2004. “Use of Microencapsulation to Prevent Acid Rock Drainage,” St. Paul, Minn.: Minnesota Department of Natural Resources.
Eger, P., and P. Mitchell. 2007. “The Use of Microencapsulation to Prevent Acid Rock Drainage,” presented at Mining and the Environment IV Conference, Sudbury, Ontario, Canada, October 19–27.
Evangelou, V. P. 1994. “Potential Microencapsulation of Pyrite by Artificial Inducement of FePO4 Coatings,”. pp. 96–103 in Proceedings, International Land Reclamation and Mine Drainage Conference and 3rd International Conference on the Abatement of Acidic Drainage, April 24–29, Pittsburgh.
Evangelou, V. P. 1996. “Oxidation-Proof Silicate Surface Coating on Iron Sulfides.” U.S. Patent No. 5494703.
Final Report: Remediation Technology Evaluation at the Gilt Edge Mine, South Dakota Mine Waste Technology Program Activity 111, Project 29. 2005.
Fytas, K., P. Bousquet, and B. Evangelou. 1999. “Application of Silicate Coatings on Pyrite to Prevent Acid Mine Drainage,” pp. 1199–1207 in Proceedings, Mining and the Environment II, September 13–17, Sudbury, Ontario, Canada.
Georgopoulou, Z. J., K. Fytas, H. Soto, and B. Evangelou. 1995. “Pyrrhotite Coating to Prevent Oxidation,” in Proceedings, Mining and the Environment, May 28–June 1, Sudbury, Ontario, Canada.
McClelland Laboratories, Inc. 2003. Update on Humidity Cell Testing. Sparks, Nev.
Metals Treatment Technologies, LLC. 2002. “Golden Sunlight Mine Acid Rock Drainage (ARD) Highwall Demonstration.”
Metals Treatment Technologies, LLC. 2003. Newsletter, Spring/Summer 03, Edition 05.
Moncrieff, V. M. 2006. The Effect and Economics of the Use of Permanganate Passivation on Acid Rock Drainage as Demonstrated in the EPA’s Multi-Cell Technology Evaluation at the Gilt Edge Mine Site. M.S. Thesis, Metallurgical Engineering, University of Nevada, Reno.
Moskalyk, R. R. 1995. “Development of a Protective Cover for Mine Waste Rock,” in Proceedings, Mining and the Environment, May 28–June 1, Sudbury, Ontario, Canada.
Roy, S., and F. Worral. 1999. “Pyrite Oxidation in Coal-Bearing Strata: The Use of P-Blocking Techniques, ” in Proceedings, Mining and the Environment II, September 13–17, Sudbury, Ontario, Canada.
USEPA (U.S. Environmental Protection Agency). 2004. Mine Waste Technology Program Remediation Technology Evaluation at the Gilt Edge Mine, South Dakota. EPA/600/R-05/002.
USEPA. 2005. Prevention of Acid Mine Drainage Generation from Open-Pit Highwalls. EPA/600/R-05/060. https://projects.itrcweb.org/miningwaste-guidance/References/prev_acid_mine.pdf.
Zhang, Y. L., and V. P. Evangelou. 1998. “Formation of Ferric Hydroxide-Silica Coatings on Pyrite and Its Oxidation Behavior,” Soil Science 163(1): 53–62.
Other Resources
CDM, 2001. Sampling and Analysis Plan for Multi-Cell Acid Rock Drainage
(ARD) Treatment Technological Evaluation, Gilt Edge Mine, Lawrence County,
South Dakota.
CDM. 2002. Multi-Cell Treatability Study Report for Gilt Edge Mine NPL Site, Lawrence County South Dakota.
Evangelou, V. P. 1995. “Potential Microencapsulation of Pyrite by Artificial Inducement of FePO4 Coatings,” Journal of Environmental Quality 24: 535–42.
Evangelou, V. P. 2001. “Pyrite Microencapsulation Technologies: Principles and Potential Field Application,” Ecological Engineering 17: 165–78.
Godin, E., ed. 1991. 1990 Canadian Minerals Yearbook—Review and
Outlook.
Ottawa, Ontario: Energy, Mines and Resources Canada.
U.S. Department of Interior. 1981. Water and Power Resource Service.
Ground Water Manual, Rev. 2, pp. 257–66.
USEPA (U.S. Environmental Protection Agency). 1991. Preparation Aids for the Development of Category II Quality Assurance Project Plans. EPA/6008/8-91/005.
USEPA. 2000. Guidance for Data Quality Assessment, Practical Methods for Data Analysis. EPA QNG-9, QAOO Update, EPN600lR-961084.
USEPA. 2002. “Final Comments to MSE Responses for Technical Systems Audit of Mine Waste Technology Program Project Prevention of Acid Mine Drainage from Open Pit Highwalls.” Memorandum (188-A4) from Lauren Drees, September 5.
Vandiviere, M. M., and V. P. Evangelou. 1998. “Comparative Testing Between Conventional and Microencapsulation Approaches in Controlling Pyrite Oxidation,” Journal of Geochemical Exploration 64: 161–76.
