Technology Overview as part of a Web-based
Technical and Regulatory Guidance
Pressure-Driven Membrane Separation Technologies
1. Introduction
Click
Here to view case study table at the end of this document.
![]()
Membrane separation technologies have been used in various industries
because of their adaptability and selectivity. As noted in the Mine Environmental
Neutral Drainage (MEND) document Application of Membrane Separation
Technology to Mitigation of Mine Effluent and Acidic Drainage (Mortazavi
2008), a membrane is essentially a thin barrier that permits selective
mass transport of solutes and solvents across the barrier. Different membrane
compositions, configurations, and characteristics are available, enabling
these processes to be used at various sites. The rate of transfer of solutes
or solvents across a membrane is controlled by a “driving force,” and the
rate of solute rejection is controlled by the size and shape of the solute
molecules (Mortazavi 2008).
Membrane separation processes have become a viable alternative to other physical methods of separation (Mortazavi 2008). For example, there is considerable need to develop separation technologies that selectively extract metal ions and radioactive species. This need is driven by more stringent environmental regulations and incentives created by the potential capture and reuse or sale of extracted metals.
While the focus of this technology overview and presentation of case studies is on reverse osmosis (RO), there are other pressure-driven membrane separation (PDMS) processes that are potentially applicable for treating mining-influenced water (MIW). The available PDMS processes are primarily distinguished from each other in terms of the molecular size of the contaminants of solutes that they will remove. PDMS processes make use of semipermeable membranes to reduce the concentration of the selected solutes in a feed solution, thus producing a permeate stream containing materials that pass through the membrane and a concentrate or waste stream, sometimes referred to as “retentate,” which contains the materials “filtered” out of the feed solution. Separation by a PDMS process results from the difference in the transport rates of various chemical species through the membrane matrix. Passage through the membrane matrix is controlled by the application of a “driving force,” which, as noted earlier, provides a basis for classifying the different PDMS processes. The types of driving forces include mechanical pressure, concentration or chemical potential, and temperature or electrical potential (Mortazavi 2008).
The characteristics of the feed solution and the desired permeate quality dictate the choice of the membrane process, the membrane type, and module design and configuration. Feed solutions with large particles and degrading organic and inorganic chemicals such as polyimide membranes are subject to degradation due to introduction of chlorine. These require pretreatment using ultrafiltration and nanofiltration, bag filters, cartridge filters, or other techniques with an RO system. Post-treatment consideration, as noted in the Kennecott Bingham Canyon case study, can include the need to remineralize permeate and perform pH adjustments. Lastly, the use of antiscaling agents to prevent scale buildup should be considered on membranes or in discharge pipes because of concentrations of calcium sulfate, magnesium carbonate, and iron sulfate compounds above their solubility.
Table 1-1 provides a good comparison of the four PDMS processes viable for MIW.
Table 1-1. PDMS processes (Mortazavi 2008)
Membrane |
Reverse osmosis (RO) |
Nanofiltration (NF) |
Ultrafiltration (UF) |
Microfiltration (MF) |
| Asymmetric | Asymmetric | Asymmetric | Asymmetric symmetric | |
| Thin film thickness | 1 micron 150 micron |
1 micron 150 micron |
1 micron 150–250 micron |
1–150 micron |
| Rejection | High and low molecular weight compounds, NaCl, glucose, amino acids | High molecular weight compounds, mono-, di- and oligosaccharides, polyvalent ions | Macromolecules, proteins, polysaccharides, vira | Particles, clay, bacteria |
| Membrane materials1 | Cellulose acetate (CA) thin film | CA, thin film | Ceramic, polysulfonic (PS), poly vinylidene flouride (PVDF), CA, thin film | Ceramic, PS, PVDF, CA |
| Pore size | <0.002 micron | <0.002 micron | 0.02–0.2 micron | 0.02–4 microns |
| Module configuration2 | Tubular, spiral wound, plate-and-frame | Tubular spiral wound, plate-and-frame | Tubular hollow fiber spiral wound, plate-and-frame | Tubular, hollow fiber |
| Operating pressure | 15–150 bar | 5–35 bar | 1-10 bar | <2 bar |
Any one of the four PDMS processes can be implemented to treat MIW, including
both surface and ground water. The primary selection criteria for determining
which process to implement should center on the treatment goal(s). If regulatory
requirements prohibit the reintroduction of treated water to the environment
or if treated water is to be provided to the public for consumptive or
irrigational use, then NF or RO are the best options to attain a quality
below federal or state maximum contaminant levels (MCLs). If less stringent
standards have to be attained, UF and MF may be more cost-effective for
treating MIW. In addition, the technology also depends on the molecular
size of the solutes requiring removal. A brief summary of the four processes
follows. s noted further, these processes have unique characteristics that
assist with separating the classes of pressure driven membrane separations
processes.
RO uses thin-film composite membranes, manufactured principally for water purification or desalination purposes, using a small pore matrix membrane. RO is a separation process like MF, UF, and NF that uses higher pressures than the other processes to force a feed solution through a semipermeable membrane with a smaller matrix pore network. “RO membranes are the tightest membranes in liquid/liquid separation” (Mortazavi 2008). This process is used to remove ionic solutes, metals, and macromolecules from feed solution and is best known from the desalination industry but has other applications such as the treatment of industrial waste water, mine water, and mill effluents (Mortazavi 2008). With the development of a new generation of membranes, such as thin-film composite membranes, which are capable of tolerating wide pH ranges, higher temperatures, and harsh chemicals such as oxidizers, the application of RO has become more widespread (Mortazavi 2008).
Water is effectively the only material that passes through RO membranes. All dissolved and suspended solutes, organic and inorganic, are rejected (Mortazavi 2008, Williams 2003). Rejection of the solutes is affected by the size and shape of the solutes, the ionic charge of the solutes, and the membrane composition and characteristics (Mortazavi 2008). In combination with an NF process, RO can cause selective solute separation based on charge, molecular weight, and size characteristics (Williams 2003). Combined with other separation technologies such as UF, evaporation, and distillation, treatment hybrids can result in highly effective and selective separation process (Williams 2003).
As noted by Williams (2003) and as included in the Kennecott Bingham Canyon case study, ideal properties of RO membranes include the following:
- resistance to chemical and microbial agents
- structural stability over long operating periods
- appropriate separation selectivity for the feed solution
1.2 Nanofiltration
(NF)
NF technology is most often used for waters with low total dissolved
solids (TDS) for the purpose of “softening,” which is the removal of
cations. NF is also used for disinfecting feed water with problems such
as membrane fouling and properties such as natural organic matter or
synthetic organic matter. Mortazavi (2008) notes that NF systems operated
at a lower pressure than that of RO systems have higher fluxes across
the membrane surfaces and the quality of permeate is lower than that
of permeate from RO systems. This process, however, has selectivity with
respect to univalent and multivalent solutes not attainable by RO processes.
NF can be used to remove smaller particles than UF and is also used in
the desalination process. Sometimes, NF is used in conjunction with another
separation method, such as RO, to ensure permeate attains drinking water
quality standards. This process can remove multivalent ions and dissolved
compounds containing sulfate, phosphate, magnesium, and calcium according
to the size and shape of the compound (Mortazavi 2008). Some information
denotes that NF can be used inexpensively as a singular treatment track
to attain a supply of clean water. Typical rejections of 60% for sodium
chloride, 80% for calcium carbonate, and 98% for magnesium sulfate are
referenced in Mortazavi (2008) for feed solutions containing 2,000 mg/L
of TDS and an applied pressure of 5 bar. Similarly to RO, pretreatment
of feed solutions and post-treatment of permeate are often necessary.
1.3
Ultrafiltration (UF)
During the UF process, suspended solids and solutes with
a high molecular weight are retained in the concentrate, while water with
low molecular weight solutes passes through as permeate. Molecular size of
the solutes removed is the primary fundamental difference between this separation
technique, MF, and NF. Solvents and salts of low molecular weight pass
through the membranes of this process, but large molecules are rejected.
Though removal of macromolecules is the selection criteria for using
this process, UF membranes have shown effective at recovering floatation
agents, surfactants and organo-metallic complexes. UF is sometimes used
as a pretreatment option for feed solutions intended to be treated by
NF or RO (Mortazavi 2008). Operating pressures for this process are less
than that for NF and RO and are necessitated, as noted in Mortazavi (2008),
to “overcome the viscous resistance of liquid permeation through the
membrane matrix’s pore network.”
1.4 Microfiltration
(MF)
MF is a PDMS process which removes contaminants from a fluid by passage
through a microporous membrane. Microfiltration has been shown to remove
major pathogens and contaminants such as Giardia lamblia cysts, Cryptosporidium oocysts,
and large bacteria. Microfiltration is typically used to separate suspended
solids (Mortazavi 2008) and can be applied to waters that are easy to
treat. Such water bodies are clear cold waters, which are potentially
susceptible to microbial contamination. The membranes of this process
typically comprise polymeric thin films with a uniform distribution of
pores and have a porosity of 80% (Mortazavi 2008). Because of the low
pressures and higher porosity of this process, the membranes are unable
to remove smaller compounds. Scott and Hughes (1996) stated this process
is characterized as “sieving” even though separation transpires as the
interaction between the membrane surface and the feed solution (Mortazavi
2008).
1.5 Other
Membrane Separation Processes (MSP)
Other MSP processes do exist (Mortazavi 2008), such as electrodialysis
(ED). ED is not a pressure-driven process but an electrochemically driven
process in which, according to Mortazavi (2008), “ions are transported
across a water-swollen ion-exchange membrane under the influence of an
electrical potential.” This process can selectively separate anions and
cations via the use of ion-exchange or ion-selective membranes. The composition
of ED membranes includes charged functional groups, chemically bound to
the membrane that can attract “counter” ions. The ED process has been predominantly
used for the desalination of brackish water, but in the mining industry
the process has been used to treat MIW and to demineralize salt or brackish
water (Schoeman and Steyn 2001, Mortazavi 2008).
2. Applicability
PDMS technologies are applicable to the following:
- MIW which includes surface water, groundwater, processed
and/or mill effluent
- high or low volume of water
- rural or urban settings and in some limited scenarios
- selective separation and precision removal for solute concentrations
- solo technology or in conjunction with others
The use of PDMS processes generally depends on the following:
- water quality restrictions placed upon the permeate (i.e., product water)
- location and source of water requiring treatment
- availability of utilities
- disposal options for concentrates (retentate)
- quality of the feed water to be treated
2.1 MIW
Surface water, groundwater, and process water such as mill effluent can be
treated using a PDMS process. The volume of feed solution factors into the
cost-effectiveness decision but does not limit the use of a PDMS process.
Low-volume or low-flow sources of feed solution may be more cost-effectively
addressed by other technologies; however, if the treated water must attain
a drinking water standard for a solute in the feed solution, a PDMS process
is likely to be more cost-effective.
At the Kennecott Utah Copper, LLC (Kennecott) Bingham Canyon Mine, years of MIW management practices and waste rock leaching activities led to the development of two large groundwater plumes (Zones A and B), called the Southwest Jordan Valley Groundwater Plumes, Operable Unit No. 2 (OU2) of the Kennecott South Zone (Figure 2-1). In Zone A, which is the larger portion of this plume, there are moderate concentrations of sulfate, ranging 500–4,999 mg/L, as the main contaminant (see the Kennecott Bingham Canyon case study). The smaller portion, or core area, of the plume has elevated concentrations of heavy metals and low pH and higher concentrations of sulfate (+20,000 mg/L). The Zone A plume core area is a 2 square mile acid plume surrounded by an approximate 10 square mile sulfate plume.
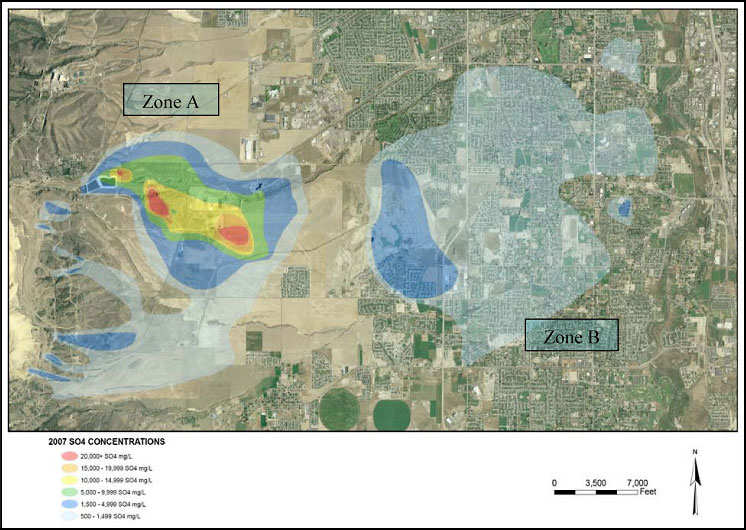
Figure 2-1. Southwest Jordan Valley groundwater plumes.
Regarding the nature and extent of contamination in the Zone A plume, while the general public does not use the aquifer as a drinking water source, there are private and municipal wells near and around the Zone A plume. Groundwater in the arid west is a source of drinking water to an ever-expanding population, and the southwest Salt Lake Valley is no exception. In 2002, the agencies selected a remedial option for the core of Zone A to address the cleanup of the groundwater with very high concentrations of sulfate, low pH (averaging 3.5), and moderate concentrations of heavy metals, including lead, arsenic, cadmium, etc. Over the same time period, the state of Utah filed a natural resource damage claim for impacts to the groundwater due to high sulfate and TDS. Utah’s primary drinking water standards are 500 1000 mg/L, respectively, for sulfate and TDS.
In 2004 the State of Utah Trustee for Natural Resource Damages entered into an agreement with Kennecott and the Jordan Valley Water Conservancy District to address the Zone A sulfate plume via RO treatment. It was agreed that permeate (or product water, which is composed of permeate with a small stream of blend water to remineralize the permeate) meeting Utah drinking water standards would be provided to the overlying affected communities. Concentrate would be disposed of into Kennecott’s operating mill tailings pipeline and ultimately in the tailings disposal facility, which is operated in compliance with permits intended to ensure protection of groundwater and surface water.
Kennecott conducted a pilot test using RO treatment plant for six years. Since then, Kennecott has operated the Bingham Canyon Water Treatment Plant (BCWTP) for 3 of the required 40-year operation (Figure 2-2). The plant was constructed on Kennecott’s property and is serviced by Kennecott’s internal power network. Pursuant to the 2004 agreement, Kennecott has, since June 2006, provided product water from the plant through the district’s distribution system to the communities in the affected area in compliance with Utah’s primary and secondary drinking water standards. The feed water is derived from two extraction wells along the leading edge of the Zone A acid plume, which is defined by the CERCLA authorities as that water with a sulfate concentration of 1,500 mg/L or greater. The feed water on average has a sulfate concentration of 1,200 mg/L and a TDS concentration of approximately 2,000 mg/L. Average yearly treatment efficiency measures have hovered around 70%–75%.

Figure 2-2. The Bingham Canyon Water Treatment Plant RO skids. Each white vessel contains seven spiral-wound membrane units. Photo courtesy of Kennecott Utah Copper, LLC.
As noted above, Kennecott elected, with acceptance from the agencies, to place the concentrates from the BCTWP into the active mill tailings pipeline. The solutes of the concentrate are very similar to the suspended and dissolved solids in the tailings slurry. The introduction of the concentrate does not adversely impact Kennecott’s ability to discharge this mixed slurry into the North Tailings Impoundment, a tailings disposal facility located in Magna, Utah, in compliance with the Utah Pollution Discharge Elimination System (UPDES) and groundwater protection permit. Other considerations at the BCTWP that can have an impact on operation and maintenance (O&M) of the BCWTP include elevated concentrations of TDS and elevated concentrations of calcium sulfate, magnesium carbonate, and iron sulfate in the feed solution and concentrate that can cause scaling on membranes and discharge pipes and biofouling.
If there is no requirement to produce permeate for public consumption, then MF, UF, or some other water treatment process such as selective precipitation, ion exchange, permeable reactive barriers, or lime treatment may be more cost-effective to treat MIW. NF, which depends on the quality of the feed water, and RO are two technologies which have the capability to produce very clean permeate from MIW but are more costly.
At the Homestake mine near Milan, New Mexico a groundwater remediation system using two PDMS units is designed to restore groundwater quality in alluvial aquifers to Nuclear Regulatory Commission (NRC) action standards. Some of the water collected from the aquifer and a large tailings pile is treated using high-pressure UF membrane. These membranes remove virtually all types of dissolved solids and concentrate them in a reject/brine solution (USEPA 2008). This system requires pretreatment through a reactor clarifier, where lime and/or caustic chemicals are added to increase the pH of the water to 10 and precipitate various salts. The water then goes through sand filters to remove particulates. Following treatment in the UF membranes, the brine solution flows to evaporation ponds for concentration (USEPA 2008).
2.2 Location and AccessibilityPDMS processes can be used in rural and urban areas, both at the source of water to be treated and in locations remote to the source (e.g., whole house residential units treating tap water). The use of PDMS processes in remote locations will be affected by the availability of power and labor; thus, this technology may not be the best option in this particular remote setting. Considerations for access to the site for constructing the treatment facility are the normal construction issues. The distance to the source feed solution is also very important. If a reliable source of feed solution is accessible, a PDMS process is feasible. If the accessibility of the feed solution is disrupted, some of the PDMS processes technologies such as RO, will require a source of water to allow for the immediate backwashing of the membranes to prevent membrane degradation.
The location of the facility also depends on a reliable power supply to operate the PDMS technology. Frequent power outages can lead to biofouling, scale buildup, and membrane degradation. This can be preventable with a redundant backwashing system for the membrane modules.
For sites where a PDMS process is intended to address groundwater, consideration must be given to acquiring permission to extract the groundwater. The location of the facility, whether in a urban, rural, or remote setting, is controlled by where an extraction point is to be located. In the western United States, extraction points are controlled by many “water-right” programs.
2.3 Separation Selectivity and Quality of Feed SolutionWilliams (2003) noted that the development of new-generation membranes is exemplified by thin-film composite membranes that are able to withstand wide ranges of pH, higher temperatures of solvents, and harsh chemical environments. These have higher water flux rates and solute separation characteristics, which has led to the wider use of PDMS processes. The selectivity of the available membranes can be used to “dialed in” on the removal of a site-specific solute or a wide range of solute species. Costello (2003) notes that the precision of solute removal is increasing with the advent of selective membranes. As noted by Mortazavi (2008), the available PDMS processes selectively address solutes of different sizes via a range of pore sizes for various membrane matrices.
Mortazavi (2008) points out that the removal of inorganic contaminants by NF and RO is the largest application of PDMS processes: “The feasibility of the application of RO and NF and even UF for the removal of hardness, nitrate, ammonia, heavy metals, and oxyanions has been demonstrated in the published literature.” Mortazavi (2008) further states that as seen during the evaluation of the Kennecott BCWTP proposal, the USEPA considers the use of RO as the best available technology to attain regulatory standards for small surface water and groundwater treatment plants.
The versatility of commercially available membranes enables designers of PDMS process systems to adapt to site-specific water qualities. The use of RO or NF is limited by the pH range where a particular membrane is stable (Mortazavi 2008). For instance, cellulose acetate membranes are not suitable for use in pH >7, while aromatic polyamide and polysulfone membranes are suitable for use in the pH range of 1–12. The versatility of the available membranes allows for the application of addressing acid mine drainage as well as treating waste streams from mills (e.g., hydrometallurgical operations).
Total suspended solids (TSS) in the feed solution can lead to clogging of the pore space of the membranes if not removed in pretreatment. Calcium, magnesium, and iron compounds can also lead to fouling of the membranes if these compounds exceed their solubility limits in the feed solution. Scale buildup from the deposition of these compounds can clog the pore spaces of the membranes or their surface area. The scale can also build up in discharge pipes, which will necessitate frequent cleanings. The solubility limits of these compounds need to be closely managed to prevent precipitation from the solute and subsequent membrane surface fouling through scale buildup. The introduction of antiscaling chemicals or adjusting the pH of the feed solution can help control the buildup of scale. As noted in the Kennecott Bingham Canyon case study and Mortazavi (2008), the introduction of oxidizing chemicals like peroxide, chlorine, and chromic acid can cause polymer membranes to degrade. Biological fouling of the membranes by bacteria or algae can occur, depending on the source of the feed water. Reducing the introduction of such biological fouling agents into the PDMS process should be included when designing such a system by the use of ultraviolet radiation to pretreat feed solution.
USEPA (2008) reported that at the Homestake mine excessive suspended solids carryover has been a problem causing blockage of the sand filters. This requires frequent backwashing. Homestake Mining Company (HMC) has switched to a proprietary flocculating agent to improve settling efficiency and reduce the suspended solids load on the sand filters. At current flow through the sand filter, the blockage issue is manageable (USEPA 2008).
The feed solution can have significant osmotic pressure that must be overcome by the hydrostatic pressure introduced into the treatment system (Mortazavi 2008): “This pressure requirement limits the practical application of membrane separation processes to solutions with total dissolved solid concentrations below approximately 25,000 mg/L.” There are some cases where this limit is not applicable, most notably disc tube applications and seawater desalination applications. “Membrane separation is a concentrating process in which the concentration of the solutes in the feed solution can be increased by several fold in the [concentrate] stream” (Mortazavi 2008). Due to increased osmotic pressure on the feed side of the membrane, this high-solute concentration could result in the reduction of the driving force [introduced pressure, hydrostatic pressure] for the separation. Such osmotic pressure buildup can in turn lead to diminished quality product water due to the increased driving force for solute transfer across the membrane and the reduced water flux.
2.4 Solo Technology or in Series with Other ProcessesPDMS processes can be designed to operate effectively as separate treatment systems or to be in series with other treatment technologies. During the treatment of acid drainage, a PDMS technology can potentially provide cost savings by reducing the volume of acid drainage to be conventionally treated by using precipitation, coagulation, or sedimentation. The treatment can thus reduce the footprint size of a clarifier (Mortazavi 2008).
PDMS processes can be used in tandem with evaporators or crystallizers which can precipitate the salts in the feed solution, thereby removing a clarifier step from the treatment system. Further treatment of the water after the precipitation of salts by a PDMS process would produce a permeate stream that could comply with most regulatory standards.
In combination with other conventional treatment processes, UF and MF are effective in removing dissolved solutes from a feed solution (Mortazavi 2008). Examples of such treatment systems include the use of powdered activated alumina to remove arsenic. RO and electrodialysis have been used to retrieve metals or plating compounds in waste waters (Mortazavi 2008).
HMC is using parallel RO membrane systems to treat contaminated water to provide low TDS water to assist in flushing a contaminated alluvial aquifer immediately downgradient from tailings piles. The treated water is reinjected into various wells, while the high dissolved solids stream is transferred to evaporation ponds (USEPA 2008).3. Advantages
PDMS technologies have the following advantages:
- long-term effectiveness
- large range of solute rejection
- tested technology and flexible application with relatively small footprint
- attainment of stringent regulatory standards
- volume reduction and waste minimization
PDMS technologies offer an effective method to produce purified water from a contaminated source. The PDMS process produces a very clean permeate and a separate brine concentrate that has the rejected solutes. These two streams do not commingle and can be effectively managed separately through the design of piping. After one pass through the process, solutes are removed from the feed solution, and the permeate water does not require further membrane treatment. However, remineralization or pH adjustment may still be necessary. The concentrate can be recirculated through the process, but recovery efficiencies will be reduced because the osmotic pressure of the feed solution is greater and the concentrated compounds are closer to exceeding their solubility limits.
If properly operated and maintained, PDMS processes can continue to function effectively over long periods. The proper application of pretreatment strategies and the periodic maintenance of the overall system help extend the life of the membrane modules. For example, following proper cleaning cycles and pretreatment strategies, the BCWTP has a contractual and design life of 40 years. The cleaning cycles have allowed the life of the membrane modules to be extended from 3 to 6 years at the Kennecott Bingham Canyon site.
3.2 Range of Solute RejectionThe use of PDMS processes in the mining industry is growing since the technologies can be used to reduce or extract metal ions and radioactive materials (USEPA 2008) from process waters and effluent. PDMS is generally a nonselective process, in that during metals removal by RO, all metals are rejected by the membrane. If there are organic contaminants, as one might find at a uranium mill operation, these too will be completely rejected. With the use of UF or MF, size exclusion can be a design parameter which will allow for selective rejection. With the use of NF separation, univalent and multivalent ions can be selectively separated to a degree. More stringent environmental protection regulations and the possible reuse of extracted metals have been an incentive for the mining industry to research the application of PDMS processes, where conventional treatment processes have less removal capabilities (Mortazavi 2008).
As noted in Mortazavi (2008):
- Conventional precipitation is not selective and is not suitable for low-concentration effluents because of their low metal recovery.
- Solvent extraction is not economical because of the loss of the extracting agent and the production of significantly larger volumes of organic wastes.
- Electrodialysis is not applicable for low concentrations.
- Ion exchange has shown some selectivity but has poor selectivity for some metal ions.
PDMS processes have shown to produce high-quality water for use not only for drinking purposes but also for industrial and commercial purposes as well. This permeate is also dischargeable with little to no impact to receiving water bodies (Kennecott Bingham Canyon case study, Mortazavi 2008, and USEPA 2008). Because of the selectivity of the membrane matrices, a PDMS processes can be geared to produce a concentrate containing a recoverable mining by-product. Because of the selectivity of the membranes, PDMS processes can be used for treatment of the following (Mortazavi 2008):
- containment pond leachate
- cooling tower blowdown
- concentrate and regeneration wastes
- high total dissolved solids effluents
- MIW drainage
- reject from reverse osmosis and nanofiltration plants
- scrubber blowdown systems
- mill and process effluents
PDMS processes are precise and can be engineered to address the characteristics of the incoming water (Costello 2003). However, such precision can be a limitation. Mortazavi (2008) documents that the higher the selectivity of the membrane, the higher the quality of the permeate yielded from the process. Hand in hand with permeate flux, which is the rate which permeate passes through a unit area of a membrane, these two properties control the mass transport property of a particular membrane. Table 3-1 denotes the separation size and permeate flux of PDMS systems (Mortazavi 2008). These are general parameters that can be affected by the type of membrane modules and matrices that are selected for use.
Table 3-1. Operational characteristics of PDMS processes (Mortazavi 2008)
Membrane |
Separation size (µM) |
Permeate flux |
| RO | <0.001 |
Low |
| NF | 0.001–0.008 |
Medium |
| UF | 0.003–0.1 |
High |
| MF | >0.05 |
High |
The RO technology does have the ability to reduce or remove the smaller solutes
in the feed solution, thus producing a very high-quality permeate.
PDMS technology has been used worldwide for purification of drinking water supplies. Portable-scale RO units have been available to campers and hikers, and the U.S. military has been developing RO systems scaled to provide water to battalions placed into the field. Residential and larger-scale systems have been used by communities to provide drinking quality water for some time. RO systems are used to treat storm water and industrial cooling systems in communities like Los Angles and other cities where there has been an increase in drought concerns. RO is used to produce deionized water, and the food industry has been using PDMS technology for the concentrating food liquids. Membrane separation processes can also be used in processing wastewater for water recovery and recycling within the metal-plating industry within the context of zero discharge.
Because of their high selectivity and thus high-quality permeate production, RO or NF have the capacity to be used as stand-alone technologies or in conjunction with another traditional water treatment method. These two PDMS processes also have the capacity to work in tandem with other technologies to meet cleanup goals. In conjunction with other treatment methods, PDMS can further treat concentrate to ensure compliance with pertinent discharge limitations.
PDMS facilities can be built with relative ease as long as access for construction equipment, personnel, and materials is provided. Such facilities can also be built for different scale sizes, depending on the source of water to be treated. Since the technology is commercially available, it is readily accessible to both industry and residential customers. Treatment facilities range in size from portable and household units, as exemplified by the case study of a long-wall bituminous mine in southwest Pennsylvania, to large-scale facilities similar to those constructed at the Kennecott site, which provide drinking water on a greater volume scale.
3.4 Attainment
of Stringent Regulatory Standards
In comparison to traditional treatment
processes, PDMS and the permeate it produces can easily meet state and federal
drinking water standards. Based on the solute sizes removed by the four PDMS
processes, most solutes that are of concern are able to be removed. The use
of RO in the drinking water production industry has been significantly reviewed,
and in most cases found to be a publicly and regulatory acceptable process
for the production of water for public consumption.
3.5 Volume
Reduction and Waste Minimization
According to Mortazavi (2008), product water
recovery rates of 80%–90% or higher have been achieved in wastewater treatment
applications using the PDMS technologies. This means that the volume of effluent
from the treatment facility is less than comparable traditional technologies
such as lime neutralization and precipitation. In conjunction with a high
solute rejection rate and the selectivity of the available membranes to extract
a wider range of solutes, more product water can be produced from the original
feed solution. With the rejection efficiencies of the available membranes,
footprints of these facilities can be smaller compared to traditional treatment
technologies. As exemplified in the ASARCO case study presented in the Mortazavi
(2008), water recovery from the PDMS/biomass media extraction/mineral media
extraction treatment system was 80%. “The concentrated water from the PDMS
stage and the metal stripping stage from the biomass polishing stage were
treated in the existing precipitation system [and] overall 85% sludge reduction
was realized”( Mortazavi 2008).
4. Limitations
- high capital and O&M costs
- requirement of osmotic pressure
- fouling of membranes and scale production
- reliance on external power
- potential difficulty of concentrate disposal
- feed solution regarding quality predictability
Depending on the water cleanup goals, these limitations can be overcome in the design of the system. In some cases where the treatment goals are less strict than MCLs, costs to construct and operate may be higher than some traditional treatment methods. However, a PDMS process is a more permanent solution and can reduce the volume of wastewater requiring further management. The following section expands on these limitations and in some cases how they can be overcome.
4.1 Higher Capital and O&M CostsPDMS processes may involve higher capital and O&M costs than to other water treatment technologies, depending on the following:
- the size of the treatment unit
- the volume of feed solution to be addressed
- the cleanup goals
PDMS processes have been demonstrated as a viable option for treating MIW, but economic limitations have slowed their widespread use in the mining industry (Mortazavi 2008). More stringent standards require higher capital costs, and long-term O&M costs may increase periodically. Though considered a limitation, these higher capital costs and ongoing O&M costs need to be compared to the production volumes of wastewater that will require further management, as well as the volumes of water that can be comparatively managed by more traditional treatment methods. PDMS processes have the capacity to produce smaller volumes of wastewater than traditional treatment technologies. PDMS process can also be designed from the beginning to address large volumes of feed solution, using a relatively smaller footprint than more traditional water treatment technologies. Thus, capital costs may be comparatively smaller and, during the life cycle, lower O&M costs may be realized. It is interesting to note that the attainment of stringent standards have placed a demand on the PDMS industry to produce more efficient and cost-effective membranes (Mortazavi 2008, Costello 2003, Williams 2003). This trend has allowed some to conclude capital and O&M costs to be a minor limitation to the use of a PDMS processes.
The volume of feed solution required to be treated, and the volume of permeate required to be produced control the size of a PDMS process facility, thus influencing the initial capital investment in these facilities. The capital costs of a PDMS process facility increase proportionally to the volume of the feed solution to be treated. However, unit costs will potentially be lower with a larger plant as the fixed costs of treatment will be divided over a larger production volume.
Smaller systems, like residential systems under sinks that treat water at individual taps, average a fraction of the capital costs which is estimated in the thousands of dollars compared to millions used at larger facilities like Kennecott’s. O&M costs of smaller systems are affected by the water quality standards that need to be attained at the spigot and the potential of unpredictable qualities of the feed water leading to more frequent replacement costs. In some cases these smaller systems may not be cost-prohibitive because of the limited volume of wastewater produced plus the wide availability and applicability of these systems.
4.2 Osmotic Pressure“In many applications, the feed solutions will have significant osmotic pressure that must be overcome by the hydrostatic pressure” (Mortazavi 2008). The hydrostatic pressure, or applied pressure, requirements limit the use of PDMS processes to treatment of feed solutions with a TDS concentration below approximately 25,000 mg/L. Disc tube applications and seawater desalination applications are exceptions to this limitation (Mortazavi 2008).
When the solute concentration rises in the feed solution, the osmotic pressure must be compensated with a driving force to facilitate permeate transfer through the membrane matrix. Permeate flux will reduce as osmotic pressure increases; thus, more and more pressure must be added into the process to ensure high-quality permeate recovery. This osmotic pressure issue will reduce the permeate recovery efficiency over time. As described during the pilot and design studies, at the Kennecott Bingham Canyon case study, the recycling of concentrate back into the RO process saw reduced permeate recovery efficiencies than when the original feed solution was put through the system. Two RO treatment skids, each with a feed solution flow capacity of 1500 gpm, are used at the plant. Each skid has two stages, where the concentrate from the first stage of the membrane pressure vessels provides the feed solution to the second stage of pressure vessels in the skid. Permeate recovery efficiencies from the recycled concentrate during the second stage were approximately 41%, compared to the average permeate recovery efficiencies of 54% in the first stage when the original feed solution was sent through the system. Pretreatment to reduce higher solute concentrations and thus reducing TDS concentrations to approximately 2,000 mg/L facilitate the use of a PDMS process but also add to the capital costs.
4.3 Fouling of Membranes and Scale ProductionMembrane fouling is probably the most significant process problem that is encountered in mining applications of PDMS and the major cause of membrane failure (Mortazavi 2008). Fouling of membranes severely impacts productivity, effluent quality, and membrane life. As described in the Kennecott Bingham Canyon case study and Mortazavi (2008), fouling can be caused by less soluble salt ions, dissolved organic compounds, colloids, fine sediments, and biological agents such as bacterium and algae. Fouling by ions in the feed solution is typically caused by calcium sulfate and ferric hydroxide.
Fouling can be controlled in the use of a PDMS process. At the Kennecott BCWTP, scaling by calcium sulfate and magnesium carbonate is controlled by the plant operators before the feed water enters the RO treatment units and afterwards because of the potential for scale to buildup in the concentrate pipeline. In the Kennecott design (KUCC 2002), gypsum saturation in the RO system is exceeded up to 700%, and silica saturation is exceeded as well. The potential for scale buildup on the membranes at the plant is controlled through the injection of a proprietary antiscalant agent produced on site, which reduces and controls the saturation rate of gypsum in Kennecott’s treatment circuit.
Fouling can be controlled by the following:
- the introduction of antiscaling chemicals (Kennecott Bingham Canyon)
- pH adjustments of the feed water (USEPA 2008)
- the use of biological controlling mechanism such as ultraviolet or chlorination if the membrane selected for use can withstand oxidizing agents like chlorine
- prefiltration systems (USEPA 2008) (Kennecott BCWTP pretreatment of the feed solution was derived from the groundwater extraction wells [KUCC 2002].)
Figure 4-1 shows pretreatment to ensure the quality of the feed solution as delineated during the design and engineering phase. The feed solution has a TDS concentration of <3000 mg/L, a sulfate concentration of <1500 mg/L, and a turbidity of <0.5 NTU. Pretreatment is accomplished through the use of UV and bag filters/cartridge filters in series. Though the feed solution is low in biological fouling agents, such as bacteria, it is first pretreated inside a UV reactor vessel to remove what fraction of microbial agents do exist to avoid clogging the pore spaces of the membranes. The feed solution is then directed to a series of pretreatment filters in two stages. The first stage of pretreatment uses vessels with polypropylene filter bags (nominal 1 µm, 100 gpm per bag) which are used to protect the cartridge filters. The second stages of pretreatment are vessels with polypropylene cartridges (nominal 3 µm, 20 gpm flow per cartridge). These pretreatment filters ensure that the quality standards listed above for the feed solution are attained and have led to the extension of the membrane lifespan at the Kennecott facility.

Figure 4-1. Kennecott’s BCTWP bag and cartridge pretreatment equipment.
Photo courtesy of Kennecott Utah Copper, LLC.
Mortazavi (2008) presents a more detailed discussion of membrane fouling, its effects, and how to control it. See also INAP (2009) for further information explaining specific mechanisms to control fouling of the membranes. As noted by the Kennecott case study, pretreatment or polishing options increase operational costs during the use of PDMS technology.
4.4 Reliance on External PowerThe mechanical system of the PDMS treatment facility, the extraction system for groundwater sources, and potentially the surface water collection and distribution system all require a reliable power supply to ensure ongoing operation. A reliable power supply includes the absence of surges in power sources or unplanned shutdowns. These factors can increase not only the obvious downtime of the overall facility but also the wear and tear on the mechanical components of the extraction systems and the plant. Unplanned shutdowns can potentially also lead to the degradation of the membranes of the plant by the introduction of fouling agents.
Groundwater extraction systems can experience wear and tear on pump motors because of surges or shutdowns, which in turn can lead to pump failures. Such shutdowns can also cause the introduction of sediments such as TSS into the PDMS systems, which can plug the pore spaces of the membrane matrices if not removed through filtration or sedimentation. These suspended solids can act as an abrasive agent on the membrane surfaces, which can lead to the degradation of the membranes and thus affect their structural integrity. Replacement costs will increase as unplanned shutdowns happen. Shutdown on surface water capture and provision systems potentially run the same risk as do shutdowns for groundwater extraction systems.
4.5 Concentrate Disposal LimitationsPDMS processes produce a concentrate that has the removed solutes in respectively higher concentrations than the original feed solution. Thus, the salinity concentration and the concentration of other metal solutes are often above typical fresh-water quality standards for receiving water bodies. The elevated metal ions and overall increased TDS concentrations limit the options for disposing of the concentrate, depending on the state and federal requirements at the site. Surface discharge of the concentrate must typically comply with fresh-water standards propagated to ensure protection of beneficial uses of the receiving surface water body. If the concentrate stream cannot comply with discharge limitations established under a National Pollution Discharge Elimination System (NPDES) permit or state equivalent, it requires some alternative discharge location or pretreatment.
In 2003–2004, the jointly proposed Kennecott and the Jordan Valley Water Conservancy District (the selected purveyor of water and joint partner with Kennecott on the overall project to address mining–related, sulfate-impacted groundwater in the Southwest Salt Lake Valley) project underwent a yearlong public comment period concerning the treatment of MIW (i.e., groundwater). Though excluded from the Kennecott Bingham Canyon case study, which focuses on the Zone A portion of the overall project, the District’s proposal for Zone B included a proposed discharge of concentrate from the second planned RO treatment plant to the Jordan River, which is a fresh-water river which discharges into the Great Salt Lake). Contaminants of concern in the concentrate from Zone B included TDS and selenium. The affects of the selenium and TDS, not only on the segment of the Jordan River where the discharge would take place but also on the Great Salt Lake, were not well known at the time. Significant public comment came in on this issue to the State Trustee for Natural Resources and the State of Utah Division of Water Quality. Such public outcry led to a six-year study of how selenium behaves in the Great Salt Lake, which is an important hemispherical stopover point for migratory birds. The result of this study was the development and ongoing propagation of the first-ever tissue-based standard for selenium for the bird population using the Great Salt Lake. The effects of the investigation and study of the Great Salt Lake environment have led to a corresponding six-year delay to the planned construction of the Zone B RO treatment plant for the Zone B sulfate plume.
The disposal of concentrate from the Kennecott BCWTP into the Kennecott mill tailings disposal system underwent an evaluation during a geochemistry study. The geochemical evaluation focused on the introduction of the concentrate from the reverse osmosis plant and the extracted acidic groundwater from the core of the Zone A plume into the tailings pipeline of the operating mill. Tailings and meteoric leach water from the mining operations is conveyed via a tailings pipeline to Kennecott’s North Tailings Impoundment located approximately 17 miles north of the site area in Magna, Utah, along the south shores of the Great Salt Lake. It was determined that the chemistry of the RO concentrate and that of clarified lime treatment overflow solutions from the lime treatment/neutralization of acidic groundwater from the Zone A plume core were chemically compatible for disposal in the Great Salt Lake and could meet the then UPDES permit discharge limitations established for Kennecott’s tailings impoundment outfall to the Great Salt Lake (KUCC 2002). However, because of the public concern over the proposed discharge from the JVWCD plant addressing the Zone B sulfate plume, Kennecott elected to discharge the RO concentrates along with the acidic groundwater extracted from the core of the Zone A plume into the tailings system rather than directly into the Great Salt Lake. The flow of concentrate from the Kennecott BCWTP represented approximately 2% of the overall flow of material in the tailings pipeline, and the solute composition of the concentrate is similar to that of the tailings from the mill. The solutes in the concentrate were found to precipitate out along the pipeline, becoming part of the solid fraction of the material in the tailings (as well as being incorporated into the scale material that builds along the inside of the pipeline). Overall the tailings management system is able to operate in compliance with the UPDES discharge limitations placed upon it. The pipeline acts as a pretreatment system prior to discharge (under permit) because the neutralization potential in the tailings is able to neutralize the pH of the extracted acidic groundwater from the core of Zone A and the RO concentrate solutes drop out of the aqueous phase. This disposal option for the RO concentrates works currently because it does not add significant costs to the overall plant operations and applicable water quality protection standards can be met at the discharge location of the tailings management system.
The management of RO concentrates in the tailings pipeline has to be done in a manner that does not hinder the disposal of mill tailings from the mine, adding an operational constraint to Kennecott’s treatment facility. Also, at the time of mine closure, Kennecott will have to reevaluate the solute composition of the concentrate to determine an appropriate disposal method.
Pretreatment of concentrate prior to its discharge into the environment can be accomplished by a number of ways and may provide a less costly means of disposal. However, depending on the degree of pretreatment necessary, the management of concentrates from a PDMS process may be cost-prohibitive. Some of the commercially available treatment options for the concentrate include lime neutralization, chemical precipitation, and vapor compression crystallization (Mortazavi 2008). These options can be used separately or in conjunction with a brine concentrator, evaporator, or a seeded-slurry falling film evaporator (Mortazavi 2008). Using neutralization or evaporators requires the use of retention structures or land disposal units that are appropriate for the characteristics of the solvents and/or solutes. Such land management structures can include (1) retention ponds, (2) landfills for the disposal of noncharacteristically hazardous solid waste, and (3) landfills for the disposal of characteristically hazardous waste. If the retention system (i.e., holding ponds or evaporation ponds) is not in an area conducive to evaporation, then the solvent retained will eventually have to be released. Such release, whether under a discharge permit (after subsequent treatment to reduce the salinity and concentration of metal ions) or into some other water treatment system, will add cost to the PDMS process.
Depending on the quality of the concentrate, it could potentially be reinjected underground as long as it is able to comply with permit limitations pursuant to federal or state regulations that cover groundwater reinjection programs. Reuse of the concentrate either for other process circuit needs (if mining facilities are still operating) or recovery of saleable products through another separation technology are alternatives to the disposal of concentrate from PDMS processes (USEPA 2008).
4.6 Feed Solution Quality PredictabilityPDMS processes can be optimized to attain the maximum water recovery between 75% and 85%. Treatment efficiency and long-term optimization of the process are affected by the quality of the feed water. Consistent and predictive water quality allows operators to design a facility that can effectively remove the solutes of concern and meet production requirements. If the quality of the feed water changes periodically on an unpredictable basis, the process will have periods where it operates effectively and periods where it does not. Membrane efficiency is affected by fouling, how often the system is washed, and the pressure differentials that build up across the membrane surfaces.
If the water quality of the feed solution fluctuates, it is possible that the pretreatment systems may not function properly and may need to be replaced more frequently, and membranes will have to be taken out of service and either cleaned or replaced. From a practical sense, if pilot studies can be performed for a period of time to evaluate fluctuations in the quality of the feed solution over a longer period, operators will be able to acquire an understanding of how best to design the PDMS process to address the potential quality issues that may arise. Realistically speaking, such preventative studies are sometimes not cost-effective. The tradeoff then becomes one of building the core system and adjusting the treatment operation with additional pre- or post-treatment systems depending on the quality of the feed solution.
Shutdowns caused by unpredicted spikes in the power supply, which then cause unpredictable solute concentrations, will hinder the ability for the process to produce required volumes, and ultimately costs will increase. Operators must ensure a reliable power supply and that the power transmission systems are protected by surge equipment and other protective equipment that guarantees “dirty power” (a term of art meaning power that has noise within it that can affect sensitive equipment) does not shutdown the mechanical systems of PDMS facilities.
4.7 Conclusion to LimitationsPDMS processes will have higher capital investment and potential improvement costs compared to other traditional water treatment technologies. Based on production volumes, ongoing O&M costs may be less per unit of product water for PDMS processes. However, O&M costs will be affected by the quality of the feed solution and the existence of fouling agents in the solution and by unforeseen power interruptions. PDMS processes will produce a concentrate that will be briny compared to most fresh-water environments, thus limiting direct disposal options without some sort of pretreatment. The selectivity of the membrane matrices, though an advantage, will also be a disadvantage because of their susceptibility to clogging or fouling through scale buildup and need for periodic cleaning. Since the osmotic pressure increases in solutions with high concentrations of solutes, solvents need to have lower TDS concentrations or higher hydrostatic pressures. Eventually recovery rates will not be as efficient if the osmotic pressure is lower.
5. Performance
PDMS processes are an effective, proven, water treatment technology. Performance
measures include the protection of human health and ecological environment
because of the prevention of contact with contamination, reduction of contaminant
migration through capture or extraction of MIW, and subsequent restoration
of MIW to premine water quality. The production of purified drinking water
can be attained as a result of PDMS processes, and solute removal can be
very effective at removing a range of solutes because of the varied membrane
matrices commercially available.
As noted previously at the Kennecott BCWTP, MIW is treated by RO. Though not directly used for public consumption, the Zone A plume has the potential to affect nearby private and municipal extraction wells if not captured and treated. Water with sulfate concentrations of 1,500 mg/L was found to have a laxative effect when consumed, which can lead to dehydration in young children and the elderly. As noted in the case study, the plant has consistently seen permeate production (or recovery) efficiencies in the range of 71%–72% during its operating period while producing a permeate cleaner, thus necessitating remineralization, than the primary and secondary drinking water standards for the State of Utah. For the 2006–2007 operating period, permeate production efficiency was 71%; for 2007 to 2008 it was 72%; and for 2008 to 2009 it was 72%. During pilot testing, permeate production efficiency for 2000 was approximately 75%; for 2001 it was 81%, and for 2002 it was 76%.
At the Pennsylvania case study site, where long-wall mining of coal affected a well and several springs, two domestic RO units were installed in 2007. At the main residence the RO unit is housed in a small shed on a concrete pad and consists of a sediment filter, RO unit, an ultraviolet disinfection unit, and a pressure tank. The second whole-house RO system was installed in the basement of the second residence and consists of a carbon filter, the RO unit, and a pressure tank. These units treat feed solution derived from two newly constructed wells installed to replace the one contaminated well and some springs that had been used to provide water for consumptive use by the two residences and livestock.
A comparison of the water quality analyses for the untreated water found concentrations of TDS exceeding Pennsylvania’s secondary drinking water TDS standard of 500 mg/L at the primary treatment unit with an average TDS concentration over a three-month period from August 2008 to October 2008 of 609 mg/L. At the second treatment unit, the average TDS concentration during the same period was 929 mg/L. At the cattle trough, the TDS concentration for sample collected on 10/28/08 was 834 mg/L. Analytical results for the treated water at the primary unit for the same time period indicated an average TDS concentration of ≤19 mg/L. One sample in October was less than the detection limit of 5 mg/L. Analytical results for the treated water at the second RO unit for August and September indicated an average TDS concentration of 88 mg/L. As to the primary unit, approximately 3% of the TDS in the original feed solution remained in the permeate. Hence, this unit had an approximate TDS removal efficiency of 97%. As for the second unit, approximately 9% of the TDS in the original feed solution remained in the permeate. Therefore, this unit had an approximate TDS removal efficiency of 91%. The ratio of treated water to wastewater is 1:4. This low production efficiency is common for the low pressures used for domestic PDMS processes and is not useful where water supplies are tighter, unlike in Pennsylvania.6. Costs
PDMS processes do involve higher costs than other
water treatment technologies. The cost depends on the size of the treatment
unit, the volume of feed solution to be addressed, and the standards that
need to be attained. The use of PDMS processes for treating MIW has been
demonstrated as a viable option, but economic limitations have slowed the
widespread use of this process in the mining industry (Mortazavi 2008). More
stringent standards will require higher capital costs up-front, and long-term
O&M costs may increase periodically.
However, the O&M costs should be compared to the production volumes to
understand how they compare to other water treatment processes.
The attainment of stringent standards have placed a demand on the industry to produce more efficient and cost-effective membranes, thus allowing PDMS processes to be considered more widely. An analysis should be done prior to selecting a water treatment process or designing a PDMS plant to determine what water quality standards need to be attained. If less stringent standards (i.e., higher than drinking water standards) need to be attained, other treatment technologies should be considered.
As Mortazavi (2008) notes, a number of general items can affect the capital costs of constructing a PDMS plant. These can include the following:
- alloys used for piping
- types of welds, pumps
- plant operating pressures
- instrumentation
- automation components
- plant configuration
The following is a list of components contributing to the capital costs associated with the construction of PDMS processes (Mortazavi 2008):
- Construction and civil engineering costs. Large plants
have higher engineering costs. Comparative to other technologies, PDMS
processes can have savings in terms of engineering costs because the plants
have small footprints that can be built on slab foundations, within standard
single-story buildings, or skid-mounted for temporary operations.
- Mechanical and electrical costs. Membrane types vary
in costs, ranging from more expensive, such as the advance ceramic membranes,
to less costly polymeric membranes. The configuration of the membranes
can also affect the costs. For smaller plants module costs might represent
10% of the system costs. Costs of the modules can increase up to 40% for
the overall system of larger plants.
- Other mechanical equipment costs. These costs can vary
depending on the selected components. PDMS processes require pumps, pipes,
valves, etc. that vary in composition and construction method depending
on site-specific conditions. On large-scale processes these costs can account
for 40%–45% of the overall system costs.
- Electrical control and instrumentation costs. For the
overall system, these costs account for approximately 10%–15% of the total
cost.
- Installation and commissioning costs. The construction of the facility is nominal about 55% of the overall costs of the system.
According to Mortazavi (2008), the primary determining factors in costing a PDMS plant are the total surface area, throughput of the system, and potential for fouling.
O&M costs vary among systems depending on the quality of the feed solution, type of membrane selected for use, frequency of cleaning, and energy consumption to operate the plant. The following is a list of components of the O&M costs associated with the operation of PDMS processes (Mortazavi 2008):
- Membrane replacement. Membrane life expectancies vary
from less than six months to over five years, depending on the quality
of the feed solution. Treating similar feed solutions, ceramic membranes
have a longer lifespan than do polymeric membranes. Longer lifespans on
membranes might be a consideration to balance the cost of these membranes.
- Energy. Pumping of water through the PDMS processes
tends to be the primary component of energy costs. As the pressure differentials
between the osmotic pressure and required hydrostatic pressure to overcome
the osmotic pressure increase, energy use increases.
- Labor. Labor is generally required to maintain the facility
and to change out the membranes when they have exceeded their usable life.
Cleaning cycles and data logging also require labor. In comparison, labor
costs for a PDMS plant can be lower than other traditional treatment technologies
because of the potential to automate some of the plant operations.
- Cleaning. Costs of cleaning chemicals and disposal of cleaning residues and wastes will account for 5%–10% of the overall operational budget.
At the Kennecott Bingham Canyon Water Treatment Plant (BCWTP), Kennecott’s treatment system was designed to provide 3500 acre-feet of municipal-quality water per year for 40 years. This system initially had a capital investment for constructing the plant of approximately $15 million dollars. Ongoing O&M costs are approximately $1.2 million per year. Labor and 24‑hour maintenance represent about 40% of this cost, which does not include the cost of operating the extraction wells. In this case, some traditional technologies had higher comparative costs, both capital and O&M, because of the projected treatment volumes. The RO system had lower costs as described in more detail in the remedial design and feasibility study for the site. It should be further noted that the capital investment costs proposed for the Kennecott RO case example were not considered insurmountable because of the available subsidy represented by a Trust Fund initially established by Kennecott and required by and in favor of the State of Utah Trustee for Natural Resource Damages as part of a settlement. For the operational time period, the use of RO was considered a viable option because of the required standards to be met, the volumes to be treated, and the lower projected volumes of wastewater that would be produced by the facility.
Cost Optimization and Other Considerations
Costs,
including capital and O&M, can be optimized
through the development and implementation of a PDMS process by considering
a number of variables. For example, the ability to predict the feed solution
quality over time and having a stable/consistent quality over time can
assist to reduce, if not at least predict, the operational costs and help
to optimize the system. Membrane selection, membrane module design, scheduling
of cleaning cycles are but a few areas where costs can be minimized if
the quality of the feed solution can be predicted and remains stable. Such
predicative ability can also assist in comparing PDMS processes to other
MIW treatment technologies to assess if there is a more cost-effective
method.
Membrane protection and optimization of cleaning cycles will assist in reducing the replacement costs of operating PDMS plants. Pretreatment costs may be relatively high or low, but the quality of the feed water is going to drive this consideration. The tradeoff for accruing these costs may be the extension to the lifespan of the membranes. Because of the planned cleaning cycles and the pretreatment units at the BCWTP, Kennecott has been able to extend the life cycle of the initial membranes to six years, three years beyond the manufacturer’s recommendation. This extension on the lifespan of the membranes has been able to reduce the O&M costs because membrane replacement is less frequent.
PDMS processes can be designed to handle various volumes and can be retrofitted at a later time if the footprint of the facility is built large enough to add further treatment skids to expand the capacity of the plant. Increasing the volume capacity of these plants later may accrue higher capital expenditures due to the costs associated with materials. Designing a plant to accommodate expansion at a later date and constructing it all at once could potentially avert higher material costs until volume expansion is needed.
The location of the PDMS plant may increase the construction costs. The costs associated with getting constructions materials and labor to a remote location may become prohibitive. A PDMS plant at a remote location will still require a power supply, which may also be cost-prohibitive. Using a PDMS process in an urban or rural setting is likely to involve less cost than in a remote location because the noted costs above are potentially less.
In addition, if the extraction or capture system for feed solution is a distance away from the actual plant, operational costs for delivering feed solution to the plant will potentially increase. For example, at the Kennecott BCWTP the operational costs of the groundwater extraction system has to account for the pumping costs to lift the groundwater from the static water level surface in each of the extraction wells to the plant (a distance of approximately 900–1000 feet). This additional lift, or pumping, adds costs associated with power use and equipment maintenance to the O&M costs of the extraction system. Constructing a plant closer to the extraction system could reduce these pumping costs.
Delivery of feed water to the PDMS facility is an operational cost that needs to be considered during design. Groundwater extraction will incur power and replacement costs over time. The distance of the extraction system for feed solutions may necessitate higher costs if vertical lifts are needed to get the solution to the plant.
Costs associated with managing permeate and concentrate vary depending on the quality of these waters, nearby receiving facilities appropriate to receive these waters, and, in the case of the concentrate, the capability to reuse this water stream. Costs to manage the permeate should on average be less than those for disposal of concentrate since permeate can be produced in compliance with drinking water standards (e.g., by RO and NF) and costs can be offset by selling the permeate to the public. Land disposal, secondary treatment, and/or discharge of concentrate can see significant costs associated with the management of this water stream. These costs include not only active treatment making use of other separation or precipitation techniques but also monitoring to ensure that the discharge of the concentrate does not cause further impact.
PDMS processes can produce product water and a smaller fraction of concentrate, or waste stream, at a volume that is more cost-effective than that of other traditional treatment methods (Mortazavi 2008). As noted in the ASARCO case study, referenced by Mortazavi (2008), the combination of PDMS followed by biomass media extraction for heavy-metal polishing and mineral media extraction for arsenic and selenium polishing was able to reduce the operational costs, which includes depreciated capital, to $15.67 (U.S.) per 1000 U.S. gallons of wastewater treated. ASARCO realized a reduction of $42.67 (U.S.) per treatment volume, compared to the original costs of a treatment system that included precipitation and pH adjustment (i.e., $58.34 [U.S.] for the same treatment volume).7. Regulatory Considerations
Regulatory constraints need to be considered as well when evaluating the
application of a PDMS process. For example, in terms of complying with the
Comprehensive Environmental Response, Compensation, Liability Act (CERCLA)
§121 and the National Contingency Plan (NCP) remedy selection criteria, regulatory
constraints include the following:
- protection of human health and the environment
- compliance with applicable or relevant and appropriate requirements
- cost-effectiveness
- using permanent solutions and alternative treatment technologies or resource-recovery technologies
- using technologies that significantly reduce the volume, toxicity, or mobility of hazardous waste, pollutants, and/or contaminants
PDMS technologies can generally comply with these criteria, as acknowledged by the 2001 Record of Decision for Operable Unit No. 2 of the Kennecott South Zone. Of the NCP criteria listed above, cost-effectiveness may not be attained if a PDMS process is selected. Post-treatment management of product water should be considered and used to drive the selection of a water treatment technology that can cost-effectively produce a water stream in compliance with applicable water quality standards.
Various federal and state regulatory statutes or programs apply to the operation of PDMS processes. This regulatory discussion pertains to the use of PDMS processes at sites within the United States. Regulatory considerations for other countries are beyond the scope of this report.
The following federal regulations or programs or the state equivalents need to be considered and permits obtained where applicable prior to implementation of a PDMS process:
- Clean Water Act (CWA) place restrictions through permits on surface water
discharges from the plant, storm-water control actions during construction
of the plant, permeate or concentrate reinjection into surrounding aquifers,
and the use of retention ponds for concentrate disposal.
- Clean Air Act places restrictions through permits on the degasifying
operations at a plant and on certain construction activities (e.g., dust
control) and established ambient air protection standards that need to
be complied with during construction.
- CERCLA and National Contingency Plan (NCP) response work has to comply
with the NCP restrictions and criteria, as well as the administrative and
enforcement requirements established under CERCLA decisions.
- Resource Conservation and Recovery Act (RCRA) places restrictions through
permits on the disposal of concentrate and potential secondary treatment
by-products, cleaning by-products, and other hazardous or nonhazardous
solid wastes from the plant, and on the use of land disposal units.
- Safe Drinking Water Act places restrictions through permits on the production and provision of drinking water to the public.
Other regulatory considerations include the following:
- 404 Permits from the U.S. Army Corp of Engineers. These permits are required
when a water conveyance or management unit is placed within, near, or through
a jurisdictional wetland or surface water body.
- Water Rights. These rights are prevalent in the western United States.
The right to extract or withdraw water, either surface or ground water,
via a point of diversion has to be acquired from state jurisdictional organizations.
- National Environmental Policy Act (NEPA). If federal funding is used to fund the construction and implementation of a PDMS process, an environmental impact evaluation must be completed pursuant to the limitations set under NEPA.
8. Stakeholder Considerations
/ Public Acceptance
The PDMS processes have been used for drinking water purification and desalination
processes on small- and large-scale practices in the United States for years.
Hence, the drinking water regulatory organizations are familiar with the
advantages and limitations of PDMS processes. The use of membrane separation
for water and waste water purification, such as the purification of storm
water in communities such as Los Angeles where industrial cooling water is
recycled, is increasing, though costs associated with large-scale plants
is still viewed as prohibitive. PDMS processes have been used in the food
industry for purification and concentration operations (e.g., concentrated
fruit juice). In the mining industry, the use of membrane separation technology
is beginning to be used to address small- and large-volume impacted sites.
As reported by Mortazavi (2008), there is “a continuous need for new separation techniques which selectively extract metal ions and radioactive species from wastewaters and industrial process streams. Stringent environmental regulations and possible reuse of extracted metals are incentives for the industry to search for new processes.” In the mining industry, the selectivity of PDMS is beginning to be viewed as beneficial to produce high-quality water for reuse or discharge (which is able to comply with regulatory standards). PDMS is also viewed as capable of producing a concentrate stream from whence valuable by-products can be extracted, then resold or reused.
Since PDMS processes are adaptable, they can be located at the “tap” in private residences as exemplified by the Pennsylvania Longwall Bituminous Coal Mine case study or at the source of the MIW, as exemplified by the Kennecott site. Though adaptable and used in areas readily accessible to the public, the general public is still not as versed on the advantages, operation, and limitations of PDMS processes. Correcting this lack would require some educational outreach.
At the Kennecott site, the proposal to use RO to treat the Zone A sulfate plume, in response to the obligations of a settlement agreement rendered in 1995, underwent public review between 2003 and 2004. The general public and environmental interests eventually were less concerned about the production of drinkable water as they were on how the concentrates would be disposed. The public comment period did take a year to get through because of the required education needed for them to understand PDMS processes.
At the site in Pennsylvania, no significant public outreach or comments period was required. The negotiation on the use of RO at the residential buildings was done between the two parties, which were the mine and property owner. Because the impact was limited to the private residence, no public involvement was necessary. What was learned by the Pennsylvania Department of Environmental Protection is that the general public is not well versed on the use of RO systems or their required maintenance. Accordingly, the use of PDMS processes at smaller sites addressing individual residences may not always be the best choice.
9. Lessons Learned
PDMS processes have been used in other industries for water purification
and wastewater treatment, and thus the capability, requirements, and limitations
of PDMS processes are known. The adaptability and selectivity of this technology
promotes its usefulness to address MIW sites. This overview supports the
conclusion that PDMS processes can produce a high-quality permeate in compliance
with stringent regulatory standards. However, the cost-effectiveness of such
PDMS systems has to be decided on a case-by-case basis.
At the Kennecott BCWTP some of the lessons learned include the following:
- Knowing the source and quality of the feed solution is critical and allows
for the implementation of pretreatment options. During pilot testing, Kennecott
observed membrane module failures caused by fine sediments in the feed
solution, which caused both fouling and abrasion, and by the brief introduction
of water with chlorine.
- Knowing the characteristic of the source water, the use of both bag filtration
units able to remove nominal particles down to 1 micron and cartridge filtration
units able to remove absolute particles size down to 3 microns in series
allowed Kennecott to design the final plant to address the abrasive and
degradation characteristics of the feed water. Understanding the source
of the chlorine allowed Kennecott to design around and prevent its introduction
into the membrane modules.
- Use of antiscalant at the start of the treatment process
allows Kennecott to reduce the buildup of solutes, which are primarily
from calcium sulfate in the feed solution, on the membrane surfaces. Kennecott
has now been able to expand the operational life of the membranes. Spiral
wound membranes are used at the BCWTP. The manufacturer recommended a three-year
lifespan on the membranes prior to replacement.
- Permeate produced at the BCWTP requires a pH adjustment after running
through the membranes. The pH of the permeate is around 5.5 and needs to
be increased to the Utah secondary drinking water standard of 6.5–8.0.
This is accomplished through the use of a degasifier which removes carbon
dioxide (the primary chemical causing the low pH). Also, the degasifier
is able to reduce radon naturally occurring in the permeate.
- The conductivity of the permeate is approximately 20–50 microSeimens
per centimeter (µS/cm). At this conductivity, the permeate can cause the
corrosion of metal distribution pipes in older municipal drinking water
systems. Remineralization of the permeate is accomplished at the BCWTP
by blending a small stream of feed solution that has been filtered and
treated through UV disinfection in with the permeate.
- The concentrate produced by the BCWTP, which represents approximately
30% of the original feed solution volume, is placed within the operating
mill tailings disposal pipeline. The pipeline delivers for disposal into
Kennecott’s North Tailings Impoundment: combined mill tailings slurry,
concentrate from the BCWTP, extracted water from the acid core of Zone
A, and other mine waters. Solids in the pipeline are permanently disposed
of in the impoundment while water is decanted and either discharged under
a UPDES permit to the Great Salt Lake or is recycled in the mine/mill process
circuit. The RO concentrate has a TDS concentration of 8000 mg/L, which
is compatible with the typical TDS concentration found in the Great Salt
Lake. However, trace metals like selenium exist in the concentrate and
were raised as a concern by the general public when the disposal of the
concentrate directly into the Great Salt Lake was originally proposed.
The selenium concentration in the RO concentrate is similar to the concentration
seen in the general mill tailings. Accordingly, the tailings system is
able to manage the introduction of trace metals while remaining in compliance
with the UPDES discharge limitations. At mine closure, disposal options
will have to be considered.
- The product water from the BCWTP is sold at a reduced rate per the terms of the 1995 settlement agreement. At this price, the product water is sold for less than the costs associated with the O&M and replacement costs at the BCWTP. What makes this project feasible is the use of trust monies that can be released pursuant to water provided from the BCWTP. It is noted from this case study that sometimes the cost to treat will be greater than what can be seen in return from the sale of the permeate. Kennecott could have used a more cost-effective water treatment technology instead of the RO treatment system addressing the Zone A sulfate-impacted groundwater if there were no need to provide quality drinking water to the affected area.
At the longwall mining site in southwest Pennsylvania, the residential treatment units were found to greatly reduce the concentration of TDS in the feed solution. What was observed, but not well understood, is that after going through the RO system, the pH levels were found to increase in the permeate from the second treatment unit. The average pH for the two sample periods was 10.4, which is above the Pennsylvania secondary drinking water standard of 6.5–8.0. Because of the nature of negotiations between the mine company and the homeowner, the Pennsylvania DEP was not directly involved with assessing how best to address the pH concerns. Some additional secondary treatment options were being considered during the required monitoring period, but final resolution on the pH concern was not made known to the Pennsylvania DEP.
Table 10-1. Case studies using PDMS technologies
Homestake Mining Company, Milan, New Mexico |
11. References
Costello, C. 2003. Acid Mine Drainage: Innovative
Treatment Technologies. U.S.
Environmental Protection Agency, Office of Solid Waste and Emergency Response,
Technology Innovation Office. www.brownfieldstsc.org/pdfs/AMDInnovativeTrtTech_03.pdf.
INAP (International Network for Acid Prevention).
2009. The Global
Acid Rock Drainage (GARD) Guide. www.gardguide.com/index.php?title=Main_Page.
KUCC (Kennecott Utah Copper Corporation). 1998a. Final Draft Remedial
Investigation Report for KUCC South Facilities Groundwater Plume, Southwest
Jordan Valley, Utah. Vers. B.
KUCC. 1998b. Final Draft Feasibility Study Report for KUCC South Facilities Groundwater Plumes, Southwest Jordan Valley, Utah. Vers. B.
KUCC. 2002. Final Design for Remedial Action at South Facilities Groundwater.
KUCC. 2005. Zone A Reverse Osmosis Plant Water System #18160: Final Design.
Mining Association of Canada. “Mine Environmental Neutral Drainage (MEND) Program.” 2007.
Mortazavi, S. 2008. Application of Membrane Separation Technology to Mitigation of Mine Effluent and Acidic Mine Drainage. Mine Environment Neutral Drainage Program (MEND) Report 3.15.1.
Schoeman, J. J., and A. Steyn. 2001. “Investigations into Alternative Water Treatment Technologies for the Treatment of Underground Mine Water Discharged by Grootelvei Mines Ltd into the Blesbokspruit, South Africa,” Desalination 133(1): 13–30.
Scott, K., and R. Hughes, eds. 1996. Industrial Membrane Separation Technology. Glasgow: Blackie.
USEPA (U.S. Environmental Protection Agency). 1996. Reverse Osmosis Process: Capsule Report. EPA/626/R-96/009. Office of Research and Development.
USEPA. 2008. Remediation System Evaluation (RSE) Homestake Mining Company Superfund Site, Milan New Mexico, Draft Final Report of the Remediation System Evaluation. Site visit conducted June 24–25.
Utah Department of Environmental Quality. n.d. “Kennecott Project” https://deq.utah.gov/legacy/businesses/k/kennecott-utah-copper/index.htm.
Williams, M. E. 2003. A Brief Review of Reverse Osmosis Membrane Technology. Williams Engineering Services Company, Inc.
______________
![]()
1 Composition of the membranes can vary between the four noted processes.
As noted from Table 3.2 of Mortazavi (2008), CA membranes are made of cellulose
acetate; PS membranes are composed of polysulfone; and PVDF membranes are composed
of poly vinylidene fluoride.
2 There are four membrane configuration types for treatment systems: plate-and-frame, spiral wound, hollow fiber, and tubular (USEPA 1996). In Mortazavi (2008), Figure 3.1 shows a typical plate-and-frame membrane system, Figure 3.2 depicts a tubular module and associated membranes, Figure 3.3 depicts a spiral-wound module, and Figure 3.4 depicts a submerged hollow-fiber module. As noted in the case study, Kennecott’s Bingham Canyon Water Treatment Plant uses spiral-wound modules in a twin-rack system.
