Case Study as part of a Web-based
Technical and Regulatory Guidance
Bingham Canyon
Water Treatment Plant
Kennecott South Zone
1. Site Information
1.1 Contacts
Mr. Douglas Bacon, CPM
State of Utah, Department of Environmental Quality (UDEQ)
Division of Environmental Response & Remediation
Telephone: 801-536-4282
E-mail: [email protected]
Mr. Kelly Payne, P.G.
Kennecott Utah Copper, LLC.
Telephone: 801-569-7128
E-mail: [email protected]
http://www.kennecott.com
1.2 Name, Location, and Description
The Bingham Canyon Water Treatment Plant (BCWTP) is part of Operable Unit
No. 2 (Southwest Jordan Valley Groundwater Plumes) of the Kennecott South
Zone (site). The site is located in the southwest section of the Salt Lake
Valley, east/southeast of Copperton, Utah. Figure 1-1 documents the proximity
of the Bingham Mining District to Salt Lake City, Utah.
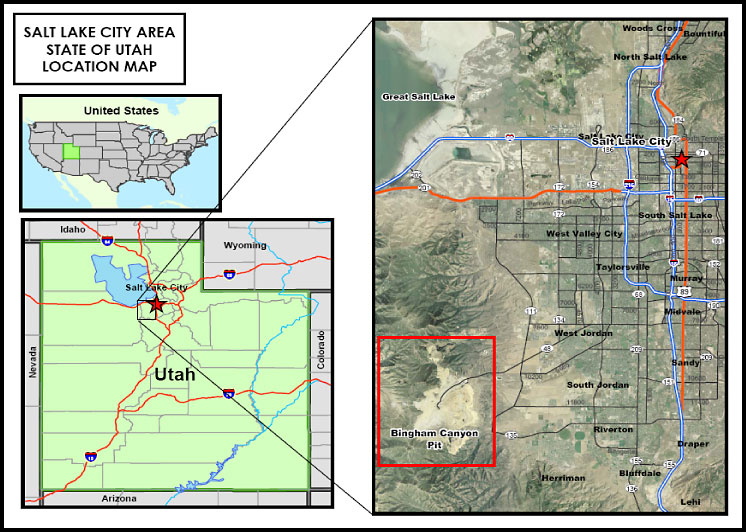
Figure 1-1. Map of the Bingham Canyon Mining District in proximity to Salt Lake City, Utah.
The Zone A Sulfate Plume (the subject of this case study) of Operable Unit No. 2 is located within the principal groundwater aquifer underlying sections of unincorporated Salt Lake County and the cities of West Jordan and South Jordan, Utah (Figure 2-1). The Utah Department of Environmental Quality (UDEQ) maintains a website on this project at https://deq.utah.gov/legacy/businesses/k/kennecott-utah-copper/index.htm, where further information is available. The UDEQ website has a document repository which includes documents on the pilot-testing and design of the treatment plant, some of which are cited in this case study.
The Zone A Sulfate Plume is located approximately 18 miles (linear aerial miles) from Salt Lake City, Utah. The Zone A Sulfate Plume underlies approximately 10 square miles and is located approximately 300 to 650 feet below the current surface grade of the Salt Lake Valley floor. The site is approximately 2 miles east of the Bingham Mining District (Bingham Canyon), an area that has been host to more than 140 years of mining, mineral processing, and active leaching operations for the extraction of copper and other precious metals from the Oquirrh Mountain area. The mining activities (i.e., extraction and milling of ore, leaching of waste rock, and more specifically the capture and management of mine-influenced water) have left a legacy of impacts upon the southwest Salt Lake Valley groundwater aquifer.
Unlined reservoirs used from 1965 to 1996 to store mine waters (i.e., barren leach water, storm water, and adit/tunnel drainage) leaked approximately 1 million gallons a day. This untreated water was the primary source to the Zone A plume (both its acidic core, delineated as the area encompassed in the colors red, orange, yellow, and green in Figure 2-1, which is not a subject of this case study, and its larger circumneutral sulfate portion delineated by the two shades of blue. Another source of mine-influenced water migrating to the valley aquifer was drainage from various historic tunnels (that was not captured) used to access the ore body or transport ore. The tunnel drainage entered the groundwater aquifer without treatment. A third source of mine-influenced water was the migration of untreated meteoric and actively applied leach water from the Bingham Canyon Mine waste rock dumps, which are located along the eastern and southern boundaries of the Bingham Mining District. This third source water migrated into the valley groundwater aquifer (by flowing along the bedrock and alluvium interface located under the waste rock dumps) prior to the development of the Eastside Collection System (a diversionary/capture system for leach waters) built in the 1990s.
2. Remedial Action and Technologies
As noted in the Kennecott Utah Copper Corporation Final Design for
Remedial Action at South Facilities Groundwater (December 2002),
“…the Remedial Investigation showed that there are about 171,000 acre-feet
of groundwater that exceed appropriate water quality criteria. The U.S.
Environmental Protection Agency (EPA) and the Utah Department of Environmental
Quality (UDEQ) have determined that the ground-water plumes containing
sulfate concentrations greater than 1500 mg/L sulfate or [low pH/heavy
metals] constitute a risk to human health and the environment.…” At the
time of this report, 171,000 acre-feet of ground water represented the
volume of impacted water in both the Zone A and B plumes (See Figure
2-1. Zone B is not the subject of this case study).
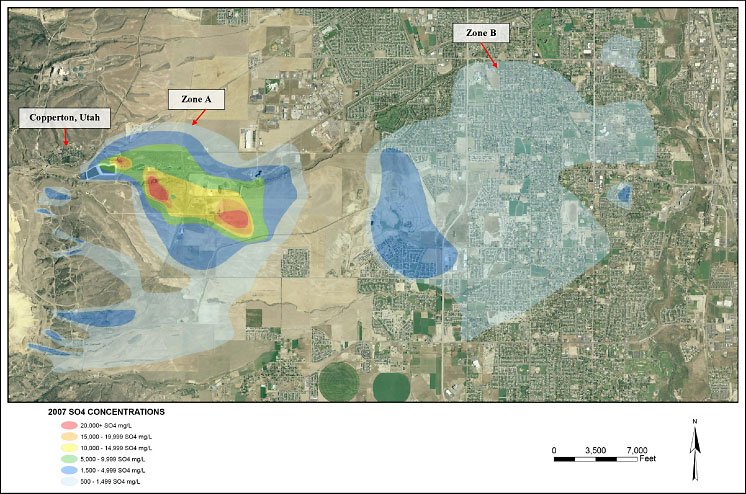
Figure 2-1. Zone A and B Groundwater Plumes of Operable Unit No. 2 (Southwest Jordan Valley Groundwater Plumes)
of the Kennecott South Zone.
In 1995 the State of Utah, Kennecott Utah Copper Corporation (now Kennecott Utah Copper, LLC; “Kennecott”) and the then Salt Lake Valley Water Conservancy District (now the Jordan Valley Water Conservancy District, “District”) settled a 1986 Natural Resource Damage claim filed by the State of Utah. The Bingham Canyon Water Treatment Plant is being used by Kennecott to treat extracted groundwater from the Zone A Sulfate Plume.
The BCWTP was constructed to address the response requirements listed in the 1995 Natural Resource Damage Consent Decree and the 2004 Natural Resource Damage Three-Party Agreement (“2004 NRD 3-Party Agreement”) rendered between three parties noted above. More specifically, Kennecott uses the BCWTP to produce 3500 acre-feet per year of water complying with the State of Utah primary and secondary drinking water standards. This is done by Kennecott to comply with the requirements for a reduction of the Zone A letter of credit held in Trust by the State of Utah Natural Resource Damage Trustee (“NRD Trustee”).
The 1995 Natural Resource Damage Consent Decree (“1995 NRD Consent Decree”) defined impacts to the groundwater aquifer in the southwest Salt Lake Valley as being injury to the groundwater where contamination caused by Kennecott (and/or predecessors) resulted in (1) increased levels over baseline of total dissolved solids (TDS), including sulfate; (2) pH levels lower than baseline; and (3) metals concentrations exceeding baseline (2 and 3 defining the core of the Zone A plume, or the Zone A Acid Plume); or (4) solid-phase contamination in the groundwater aquifer that can be redissolved in the future. The overall response work being implemented by Kennecott at Zone A is addressing the two portions of this plume:
- Zone A Acid Plume: An area where the pH is approximately 3.5, sulfate concentration ranges from 1,500 mg/L to greater than 20,000 mg/L, and metals concentrations (i.e., arsenic, cadmium, lead, selenium, chromium) are above Utah’s primary drinking water standards. Remedial action (not a subject of this case study) is being implemented in compliance with the selected remedy under CERCLA (2001 Record of Decision), the Clean Water Act, and the 1995 NRD Consent Decree.
- Zone A Sulfate Plume: An area where the groundwater is circumneutral with sulfate concentrations ranging from 500 mg/L (UDEQ’s primary drinking water standard) to approximately 1,500 mg/L. The response action (the subject of this case study) is being implementing in compliance with the terms of the 1995 NRD Consent Decree and 2004 3‑Party Agreement (as noted above), CERCLA and the Clean Water Act, and the UDEQ propagated drinking water standards, as allowed under the Safe Drinking Water Act.
Reverse osmosis (RO) is being used at the BCWTP as the primary technology for addressing the TDS and Sulfate impacted ground water extracted from the Zone A Sulfate Plume. The BCWTP has two RO treatment racks where groundwater with a moderate TDS concentration of ≈2000 mg/L and sulfate concentration of 1,200 mg/L (once extracted via three extraction wells, “Barrier Wells”) is treated. Spiral-wound RO membranes are used at the BCWTP (Table 2-1). The BCWTP has been in pilot- or full-scale operation since 2003 (“complete and operational status” was granted by the NRD Trustee on July 26, 2006). From 2000 to 2002 (pilot stage), the BCWTP treated 993 acre-feet of impacted groundwater. From June 2006 to May 2009 (operational stage) the BCWTP treated 13,338 acre-feet of feed water (June 06 to May 07: 4710 acre-feet; June 07 to May 08: 4141 acre-feet; June 08 to May 09: 4487 acre-feet).
The BCWTP is permitted by the UDEQ Division of Drinking Water for the production of municipal-quality water (drinking quality water defined under the 1995 NRD Consent Decree) to be consumed by the public in the affected communities. The BCWTP’s discharge of concentrate or wastewater is directed (permitted by rule) to Kennecott’s tailings pipeline, which directs commingled mill tailings, mine-influenced waters, acid water extracted from the Zone A Acid Plume, and RO concentrate to Kennecott’s North Tailings Impoundment operated in compliance with the groundwater and surface water protection permits issued by the UDEQ Division of Water Quality.
The footprint of the BCWTP is 14,600 square feet (Figure 2-2). The treatment system at the BCWTP comprises (1) extraction and conveyance system: three extraction wells and associated pipelines, feed water tanks and pipelines, product water conveyance pipelines, and associated meters; (2) pretreatment system: bag and cartridge filters rated to 45 microns, ultraviolet system to prevent the biological fouling of the membranes from bacteria, and antiscalant system which injects a proprietary antiscalant compound used to prevent the scaling of gypsum on the membranes; (3) the RO membrane system (Figure 2-3); (4) a remineralization system where some feed water (once treated with UV) is blended with the RO permeate to remineralize the permeate; and (5) degasifying system used to reduce the concentration of carbon dioxide (which helps to increase the pH of the product water1) and radon in the product water. Table 2-1 provides some general information about the BCWTP on the RO membranes and performance capabilities.

Figure 2-2. The Bingham Canyon Water Treatment Plant.
(The overall facility site is 2.5 acres; the BCWTP itself is 14,600 square feet.)

Figure 2-3. Two reverse osmosis treatment racks of the BCWTP. The white vessels in the racks contain the spiral-wound RO membranes. Each rack contains two stages: Stage 1 contains six columns of RO vessels, Stage 2 contains 3 columns of RO vessels.
Table 2-1. BCWTP Facts
Configuration |
2 two-stage treatment racks |
Feed rate |
3000 gpm to membranes 200 gpm to remineralization 3200 gpm total |
Membranes |
Hydronautics ESPA2 (spiral wound polyamide) |
Pressure vessels |
Protec and CodeLine |
Permeate recovery rate |
71%–74% |
Production |
2200 gpm permeate 2400 gpm product |
3. Performance
Literature research (completed during the Remedial Investigation done
under CERCLA oversight for Operable Unit No. 2 of the Kennecott South Zone)
assessed that waters with a sulfate concentration of 1500 mg/L can have
a dehydration effect upon the general public (more so for young children
and older adults) because of the onset of diarrhea. UDEQ’s primary and
secondary drinking waters standards are propagated to protect public health
and to affect the aesthetic qualities of water provided for consumptive
use by the general public. As it pertains to sulfate, the primary drinking
water standard is designed to minimize the noted health effect and the
side effect of dehydration (the secondary standard addresses the color
and smell of drinking water). Utah’s primary drinking water standard for
total dissolved solids (TDS) is 1000 mg/L and for sulfate is 500 mg/L (sulfate
is a component of TDS). Under the 1995 Natural Resource Damage Consent
Decree the standards established for TDS and sulfate in the product water
are the same as the secondary drinking water standards of the State of
Utah (Table 3-1).
Table 3-1. Treatment goals
Contaminant |
Cleanup Concentration |
| Total dissolved solids | UDEQ’s secondary drinking water standard: 500 mg/L2 |
| Sulfate | UDEQ’s secondary drinking water standard: 250 mg/L |
Pursuant to an agreement rendered between Kennecott and the District (“2004
NRD Project Agreement”) for the project, Kennecott added treatment enhancements
to ensure that the quality of product water complied with a TDS concentration
of 250 mg/L and a sulfate concentration of 250 mg/L (the District pays for
this treatment enhancement). The District wanted the product water from the
BCWTP to have a quality similar to the District’s other drinking water sources
that feed into its distribution system. Because it is chemically impossible
for sulfate concentrations to exceed TDS concentrations (because sulfate
is a component of TDS), Kennecott’s compliance with the TDS criterion for
product water (see Table 3-2 for yearly averaged TDS concentrations in product
water) ensures compliance with the sulfate criterion of the 2004 NRD Project
Agreement.
Table 3-2. Average TDS concentrations in product water
Operating/reporting
period |
Averaged
TDS concentration in permeate from
the Bingham Canyon Water Treatment Plant3 |
| Sept. 2006–Feb. 2007 | 222 mg/L (average of two grab sample data results) |
| Sept. 2007–May 2008 | 221 mg/L (9 month average of grab sample data, including the average of 5 samples collected in October 2007) |
| June 2008–May 2009 | 242 mg/L (monthly grab sample for 12 month operating period) |
The BCWTP has consistently seen permeate production efficiencies in the range
of 71%–72%. For the 2006–2007 operating period (June 1st–May 31st), permeate
production efficiency was 71%; for 2007–2008, 72%:, and for 2008–2009, 71%.
In terms of industry standards for RO membrane efficiencies (relevant to
the production of treated water), the efficiencies seen at the BCWTP for
the first three years of operation are comparable. Compared to permeate production
efficiencies seen during pilot testing (Table 3-3), the operational efficiencies
are comparable.
Table 3-3. Permeate production efficiencies during pilot testing
Pilot year |
Efficiency4 |
2000 |
74% |
2001 |
81% |
2002 |
75% |
The lifespan of the RO membranes at the BCWTP have been increased by Kennecott
because of their understanding about the quality of the feed water and scheduling
of periodic cleaning cycles. The manufacturer of the membranes recommended
a lifespan of three years, with periodic cleaning cycles. Kennecott’s planning
and designing of the treatment system and optimizing operational activities
around the quality of the feed water has allowed Kennecott to realize approximately
six years of operational life on the RO membranes.
Another measure of efficiencies at the BCWTP includes TDS removal efficiency. During pilot testing (Table 3-6 in the Kennecott Utah Copper Corporation document entitled Final Design for Remedial Action at South Facilities Groundwater, December 2002), the approximate TDS concentration of the feed water was 2270 mg/L. Permeate had an approximate TDS concentration of 33 mg/L; thus a TDS removal efficiency of 98.5% was observed during pilot-testing of the RO membranes tested. Because RO membranes separate solutes at the molecular level, pilot-testing observed the removal of metals from the feed water at comparable efficiencies as TDS and sulfate (though the concentration of metals were lower in the feed water).
During the past three operating years, TDS removal efficiency was assessed using specific conductance as a surrogate. As noted in Table 3-4, removal efficiencies at the BCWTP averaged 98.9%. Compared to industry standards, the RO membrane modules at the BCWTP are highly effective at removing TDS (and as a result sulfate as well).Table 3-4. TDS removal efficiencies for the first three years of reported operations
Operating/reporting period |
TDS removal efficiency for
the Bingham Canyon Water Treatment Plant membranes |
June 2006 – May 2007 |
99.1% |
June 2007 – May 2008 |
98.9% |
June 2008 – May 2009 |
98.8% |
Tables 3-5, 3-6, and 3-7 document the average TDS and sulfate concentrations
within the feed, permeate, and concentrate water streams at the BCWTP. The
water quality data is from samples collected and analyzed from January 2009
to April 2010 (in the case of the monthly samples for permeate and concentrate
some monthly samples were missed). Quarterly samples of the feed water were
collected. Monthly samples for permeate and concentrate were collected. These
three tables demonstrate (using concentration) the removal efficiency of
the RO membranes at the BCWTP.
Table 3-5. Average TDS and sulfate concentrations in feed water5
Extraction well |
Sample population |
Average TDS concentration (mg/L) |
Average sulfate concentration (mg/L) |
B2G1193 |
N=7 |
3101 |
1901 |
BFG1200
|
N=5 |
1472 |
716 |
LTG1147 |
N=6 |
1485 |
565 |
Table 3-6. Average TDS and sulfate concentrations in the permeate water6
Extraction well |
Sample population |
Average TDS concentration (mg/L) |
Average sulfate concentration (mg/L) |
| Rack 1 | N=14 |
<22 |
<5 |
| Rack 2 | N=16 |
<25 |
<7 |
Table 3-7. Average TDS and sulfate concentrations in the concentrate water7
Extraction well |
Sample population |
Average TDS concentration (mg/L) |
Average sulfate concentration (mg/L) |
| Rack 1 | N=13 |
8392 |
4733 |
| Rack 2 | N=16 |
8564 |
4782 |
For the past three operational years (June 1st to May 31st, for 2007, 200,8
and 2009) product water has consistently complied with all applicable State
of Utah primary and secondary drinking water standards, and continues to
remain in compliance with permit limitations established by the State of
Utah Division of Drinking Water. Figure 3-1 provides a graph of the volume
of product water (in acre-feet) for the first three years of the operational
period. As noted from the 2004 NRD 3-Party Agreement, Kennecott must supply
3500 acre-feet per year (on a five-year rolling average) for 40 years; 2011
will be the first year this compliance measurement will be made (“YE” stands
for Year End).
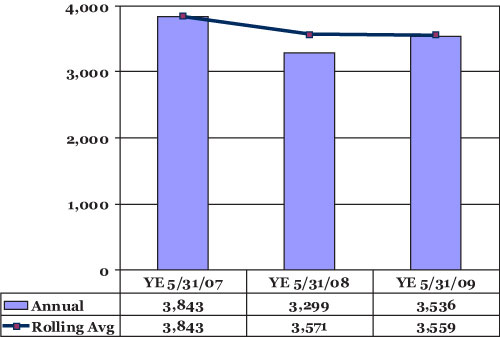
Figure 3-1. Graph of year-end production volume totals at the BCWTP for the first three years of operations.
4. Costs
The
BCWTP was designed (and is operated) to produce (on average) 3500 acre-feet
per year of treated product water for a 40-year period (Figure 3-1). The
capital and yearly operation and maintenance (O&M) costs are presented
as total costs, which do not represent the costs associated with extraction
wells, feed pipelines, and disposal infrastructure.
- Total capital costs for the BCWTP: approximately $15.0 million (U.S.)
- Total yearly O&M costs (40% of these costs represent labor and 24-hour maintenance) for the BCWTP: approximately $1.2 million (U.S.)
5. Regulatory Challenges
No significant regulatory barriers have been encountered with the use of
RO to date at the BCWTP. Use of RO to produce drinking water in compliance
with standards under the Safe Drinking Water Act (or comparable state programs)
has been previously recognized (in the drinking water production industry)
by regulatory agencies as a useful technology.
Pertinent to this case study, the regulatory barriers that have previously arisen and may again in the future stem around the discharge of concentrate (i.e., wastewater or the brine stream) from the BCWTP once the current option (i.e., placement into Kennecott’s mill tailings pipeline and ultimately in the North Tailings Impoundment) becomes unavailable. The salinity concentration of the concentrate will be relatively high compared to Utah water quality standards for surrounding freshwater surface bodies. Selenium (a trace constituent) may also have a concentration relatively high compared to applicable water quality standards for the surrounding freshwater surface bodies (where a discharge might be directed).
The salinity concentration of the RO concentrate (approximate TDS concentration of 8000 mg/L) is less than the TDS concentration in the Great Salt Lake (80,000–120,000 mg/L). Thus, a discharge directly to the Great Salt Lake of RO concentrate from the BCWTP may be permittable under Utah’s UPDES program. Selenium concentrations in the RO concentrate (14 mg/L, Table 3-6 of the December 2002 Kennecott Utah Copper Corporation document entitled Final Design for Remedial Action at South Facilities Groundwater) might limit discharge options to the Great Salt Lake8. It is noted that this selenium concentration is currently dischargeable to the Great Salt Lake because it is within the limits set under Kennecott’s current UPDES permit. As other numerical standards begin to be adopted for protection of the Great Salt Lake, these too may limit discharge options without pretreatment of the concentrate to reduce the contaminants of concern (if the intention is to direct the RO concentrate to the Great Salt Lake).
6. Stakeholder Challenges
The general public and other interested parties were engaged through a public
comment period when the use of RO membrane separation to produce municipal-quality
water from the Zone A Sulfate Plume was proposed to the NRD Trustee. The
use of RO membrane separation to treat the Zone A Sulfate Plume groundwater
was not a significant concern of the public and other interested parties.
However, some in the general public sector were misinformed and thus concerned
about the source of feed solution to the BCWTP (in terms of the capability
of the BCWTP to produce drinking quality water). This concern arose because
of the belief that the Zone A Acid Plume (with the low pH and heavy metals)
was going to be treated and provided as drinking water and the public did
not understand the capability of RO membranes to remove the perceived elevate
metals concentrations in the feed water. It should be noted that the trace
metals in the Zone A Acid Plume and Sulfate Plume are not considered hazardous
under RCRA but do exceed Utah’s primary drinking water standards. Once it
was understood that the feed water would be derived from the Zone A Sulfate
Plume (containing some trace metals, but primarily elevated TDS and sulfate)
and that the product water would be in compliance with the primary and secondary
drinking water standards for all applicable contaminants of concern, the
general public generally accepted the use of RO membrane separation at the
BCWTP.
Some concerns about taste and hardness of the product water were raised by the public. However, since 2006 these concerns/comments have not been raised by the public. The discharge of concentrate was and continues to be a different concern of interest.
The delivery of concentrates from the BCWTP to Kennecott’s mill tailings pipeline and eventually into Kennecott’s North Tailings Impoundment for final disposal was judged inappropriate by some members of the public and environmental interest groups. These stakeholders were concerned that the proposed disposal option was simply a postponement of final treatment of the contaminants of concern. Their concerns consistently alluded to the creation of a new contaminant plume in an area previously not impacted by the plumes at the Site9. Once it was understood (1) how Kennecott’s North Tailings Impoundment (NTI) operates, (2) how the contaminants of concern are settled out in the NTI and permanently sequestered, and (3) that the NTI was operated in compliance with the limitations set by State of Utah Division of Water Quality for groundwater and surface water protection, some of the stakeholders no longer had this concern.
To educate the stakeholders, the NRD Trustee (with assistance from Kennecott and District) spent two years performing outreach and education during an extended public comment period. Approval of the project was finally granted by the NRD Trustee in 2004.
The discharge of concentrate from the District’s planned reverse osmosis plant to treat Zone B Sulfate Plume water (not a subject of this case study but part of the overall cleanup project) led the State of Utah to initiate a six-year study to develop a first ever numerical water quality standard (tissue based) for selenium (for the Great Salt Lake). Concerns were raised about the lack of understanding the regulatory agencies had on how selenium would respond (i.e., would it be sequestered?) in the hyper-saline environment of the Great Salt Lake and how it would potentially be available to the biota of the GSL environment. The interested stakeholders felt a need to increase the knowledge of how the physical and chemical properties of the Great Salt Lake might affect the bioavailability of selenium in the concentrate before any discharge under a UPDES permit should be granted. This concern was not initially anticipated by the regulatory group but was quickly recognized and ultimately was part of the consideration made when the project was approved by the NRD Trustee.7. Other Challenges and Lessons Learned
An effective industry/public/regulatory working group has been the key to
success for this project. Regular meetings and annual reporting to the Kennecott
South Zone Technical Review Committee (TRC) built a remarkable amount of
credibility and trust between all parties involved with the project. Subsequent
to the public comment period on the project to treat Zone A Sulfate Plume
groundwater, the Trustee brought together another community based stakeholder/outreach
group with a focus on providing a nontechnical overview of the project and
its continued implementation. Both groups have been in the past (and continue
to be) kept informed about the progress of cleanup.
It is interesting to note that some members of these two groups (TRC and the Trustee’s Stakeholder Forum) hold membership on both groups and were involved with the UDEQ Division of Water Quality’s efforts to develop the selenium numerical water quality standard for the Great Salt Lake. Receiving a consistent message and being involved with the multiple facets of the overall project have facilitated the subsequent agreement on the project by stakeholders previously critical about the project. As a result of the educational/outreach efforts, there has been a willingness on the part of all interested parties to carefully consider recommendations pertaining to the implementation of the project and render constructive (rather than antagonistic) feedback.
In terms of lessons learned about RO membrane technology, this case study has taught the parties involved that the design of a RO membrane plant needs to focus on the quality of the feed water. Early membrane failures (in terms of structural integrity) caused by the introduction of abrasive fine sediments and chlorine10 had to be controlled. Fine particles were controlled through the design of bag filter and cartridge filter units in series prior to delivery of feed water to the membrane racks. As a result of the pilot studies and the inadvertent introduction of chlorinated water to the RO membranes, Kennecott learned to select membranes for the RO system able to withstand (to a degree) the introduction of chlorinated water. The use of an antiscalant solution at the front end of the treatment process was found to assist with the prevention of solute buildup along the membrane surfaces (scale arising from the concentration of calcium sulfate, magnesium carbonate and iron sulfate in the feed water). Permeate was found to have a low pH (≈5.5) after treatment, thus requiring adjustment. Adjustment of the pH is accomplished through the use of a degasifier capable of striping carbon dioxide (primary cause for the lower pH), as well as the introduction of sodium hydroxide into the product water. Interesting to note, the degasifier also addresses the removal of radon natural occurring in the feed solution.
Finally, another lesson learned was that permeate has to be remineralized. Permeate from the RO membrane racks has a conductivity of approximately 20–50 µS/cm, which can corrode metal pipes of older public distribution systems. Remineralization is accomplished by Kennecott through blending a small volume of by-pass water11 into the permeate stream after the membrane treatment. Product water continues to remain in compliance with the state’s primary and secondary drinking water standards and has an average TDS concentration close to 250 mg/L to prevent corrosion of the conveyance pipes of the District’s distribution system.
8.0 References
Annual Report on Zone A Plant Operations and Acid Plume Extraction,
Letter from Kennecott Utah Copper Corporation, September 27, 2007.
Annual Report on Zone A Plant Operations and Acid Plume Extraction, Letter from Kennecott Utah Copper Corporation, July 31, 2008.
Annual Report on Zone A Plant Operations and Acid Plume Extraction, Letter from Kennecott Utah Copper Corporation, July 29, 2009.
Final Design for Remedial Action at South Facilities Groundwater, Kennecott Utah Copper Corporation, December 2002.
Proposal to the Utah State NRD Trustee and US EPA CERCLA Remedial Project Manager for a Groundwater Extraction and Treatment Remedial Project in the Southwest Jordan Valley, Kennecott Utah Copper Corporation & Jordan Valley Water Conservancy District, June 11, 2004.
South Facilities Groundwater Construction Completion Report, Kennecott Utah Copper Corporation, December 2006.
South Facilities Groundwater Remedial Action Progress Report 2006, Kennecott Utah Copper Corporation, March 2007.
South Facilities Groundwater Remedial Action Progress Report 2007, Kennecott Utah Copper Corporation, May 2008.
South Facilities Groundwater Remedial Progress Report 2008, Rio Tinto–Kennecott Utah Copper, April 2009.
State of Utah Natural Resource Damage Trustee, Southwest Jordan Valley Groundwater Cleanup Project/State of Utah Natural Resource Damage Trustee Comment Response Summary, August 31, 2004.
_______________________
![]()
1 “Product water” is a term
used throughout this case study to reference the final water that is provided
to the District for provision to the communities. It is a blend of permeate
from the treatment system and a small volume of by-pass water (for definition
see footnote 11) in compliance with the State of Utah’s primary and secondary
drinking water standards.
2 The 1995 Natural Resource Damage Consent Decree defines municipal quality water, the cleanup standards for the project, as water with chemical concentrations at or below 250 mg/L sulfate and 500 mg/L total dissolve solids for the area west of the Welby Canal or 250 mg/L sulfate and 800 mg/L total dissolved solids for the area east of the Welby Canal and which otherwise meets primary drinking water standards for other contaminants.
3 Sampling strategy and reporting evolved during the initial startup of the project. Ultimately under agreement reached between the State of Utah Trustee for Natural Resource Damages and Kennecott the operating period (hence the reporting period) was established as June 1st to May 31st. Formal reporting on product water quality caught up with the operating cycle in the 2009 report..
4 Efficiencies were calculated from data presented in Table 3-5 from the December 2002 Kennecott Utah Copper Corporation document entitled Final Design for Remedial Action at South Facilities Groundwater. The slight variation in calculated numbers is due to rounding.
5 The feed water is a composite stream of water extracted by the three extraction wells. Each contributes a percentage of the overall volume of the feed water: B2G1193: 41%, BFG1200: 41%, LTG1147: 18%. The overall TDS and sulfate concentrations in the combined feed water can be adjusted by changing the contributory percentages of the three extraction wells.
6 On average 75% of the TDS and sulfate data set comprised of data points less than the Method Detection Limit (<MDL). As such, the MDL (as a whole number) was used to calculate the average TDS and sulfate concentrations presented in Table 7.
7 Average concentrations presented in Table 8 are approximations because of the method of rounding the resulting concentration to avoid the decimal.
8 Such is dependent on the final numerical quality standard, a tissue-based standard, yet to be accepted by EPA Region VIII but adopted under rule by State of Utah Division of Water Quality, UPDES section.
9 It should be understood that this concern/critique was raised primarily against the treatment option proposed for the Zone A acid plume but was also leveled against the disposal of reverse osmosis concentrates as well.
10 Kennecott does not add chlorine directly into the treatment system to prevent biological fouling; it uses primarily UV and can make use of a nonchlorinated biocide. The chlorine that was introduced into the membrane treatment system (during pilot studies) was introduced inadvertently. The source of the water with the chlorine has subsequently been prevented from entering the treatment system.
11 By-pass water is derived by bleeding off a small volume of feed solution, prior to treatment by the RO system, and treating the by-pass water via a sediment filter and UV. The by-pass water complies with the appropriate drinking water standards of Utah and is used to primarily increase the TDS of the permeate, thus increasing the conductivity of the permeate as well.
